The 20-year activities of a medical supervisory panel appointed under the terms of a settlement agreement of the Bowling v. Pfizer class action suit involving the Björk-Shiley convexo-concave (BSCC) heart valve are detailed. Of approximately 86,000 valves implanted, catastrophic failure of the valve was reported in 663 patients from 1978 to 2012. In 1994, a 7-member medical panel consisting of cardiologists, cardiovascular surgeons, epidemiologists, and a nontechnical chairman was appointed by the federal court. The panel collected clinical and manufacturing data, supported epidemiologic studies assessing risk factors for valve fracture, and developed guidelines for payment for explanting potentially defective valves in patients. Three sets of guidelines, based on comparisons of estimated risks of valve fracture versus risks of valve replacement surgery, were issued by the panel to help guide patients and their physicians in decisions about explanting valves. In addition, the panel supported research directed at identifying valves at risk for outlet strut fracture. The primary techniques evaluated included analyzing acoustic signals from the valves, imaging valves for potential cracks in the struts, and structural analyses of Björk-Shiley convexo-concave valves, but none proved applicable for large-scale surveillance of the patient population. The panel also became a patient advocate and acted as an intermediary between the manufacturer and the attorneys initiating the legal settlement. The panel’s experiences may help inform future strategies for guideline development for other medical devices or procedures involving risk-benefit comparisons.
The past 1/2 century has witnessed enormous advances in the development and refinement of implantable medical devices such as heart valves, joint replacements, and pacemakers/defibrillators. For the most part, these devices have improved the quality and, in some cases, the duration of human life. Unfortunately, occasional unexpected adverse events have and will continue to occur (e.g., the recently reported problems with hip replacements and cardiac pacemaker/defibrillator leads), with the result that very careful monitoring of new devices is now recognized to be an essential component of the evaluation of health services and the protection of patients. Fracture of the outlet strut of the Björk-Shiley convexo-concave (BSCC) heart valve was one of the first and most catastrophic of these adverse events.
The BSCC valve, originally released in 1978, was approved by the Food and Drug Administration (FDA) in 1979, after which large numbers were distributed by Pfizer Inc., for implantation worldwide. During the next several years, multiple instances of catastrophic failure of the BSCC valve, often with patient death, were reported. These reports resulted in numerous wrongful death lawsuits. A class action lawsuit, known as Bowling v. Pfizer, was filed in 1992, the principal purpose of which was to deal with class members’ fear of further injury or death.
The settlement agreement provided for implantees to be members of the class and receive certain benefits, unless they opted out. It also provided that a supervisory panel (the panel) of experts would be appointed for the purposes of (1) establishing and modifying guidelines under which monetary benefits would be paid to class members who elected to have their BSCC valve(s) explanted, (2) conducting “research and development of diagnostic techniques to identify implantees who had significant risk of strut fracture”, and (3) “conducting research concerning the characterization and/or reduction of the risks of valve replacement surgery, including improvement of the techniques for such surgery”. The panel was appointed and began its work in 1994; it consisted of 7 members, 6 of whom were medical doctors who were experts in cardiology, cardiovascular surgery, and epidemiology and a nonscientist chairperson who organized the efforts of the panel. A significant sum of money was committed under the supervision of the federal court to support the research mandated in the settlement, which was unique at the time for a settlement of this sort.
Our purposes in this publication are to (1) summarize the panel’s activities over the 20 years of its existence, (2) review the history and current status of the BSCC heart valve issue, and (3) highlight those lessons that may be useful to other similar panels tasked with assessing the risk and promoting research into failures of other medical technologies that might be implanted in patients.
Background
The BSCC valve
The BSCC tilting disc valve was designed to replace the original radial spherical (R/S) model, which was prone to valve thrombosis. The BSCC heart valve was considered an improvement over the earlier version because its strength was increased by making the inlet strut part of the orifice ring and doubling the cross-section area, thus improving hydrodynamics and eliminating the area of stagnant and low flow behind the disc (thereby decreasing the thrombosis risk). The valve also contained an outlet strut made of Haynes 25 alloy (M. Vincent and Associations, Ltd., US Distribution, Inc., Windfall, Indiana) whose legs were welded into the valve ring. The tip of the outlet strut was bent downward to form a rounded hook that fit into a circular depression on the downstream side of the disc (the well) and served to hold the disc in the valve housing as it rotated between the open and closed positions ( Figure 1 ). Valve closure occurred in 2 phases: translation, in which the disc moved backward toward the valve ring, terminating when the edge of the occluder impacted the ring, followed by rotation about this axis to the closed position. By design, the axis of rotation was close to the base of the outlet strut so that the major force of the rotational impact was applied to the larger molded inlet strut, with any slight overrotation impacting the base of the outlet strut. All the initial valves produced had a 60 ° opening angle; a second version of the valve with a 70 ° opening angle was introduced >1 year later and was frequently implanted in Europe, but it was never approved for implantation in the United States (US).

Worldwide, nearly 86,000 valves (54% aortic and 46% mitral) were shipped and not returned and, therefore, were assumed to have been implanted. More than 31,000 were in the US; other countries with significant numbers of implanted valves included Canada, England, France, Germany, India, Italy, Japan, Spain, Sweden, and the Netherlands.
Outlet strut fractures
The first fracture of the outlet strut of a 60 ° valve occurred during clinical trials in 1978; as increasing numbers of valves were implanted, additional reports of similar fractures were received by the manufacturer. Although the number of fractures was small compared with the number of valves implanted, the fact that they all occurred near the weld site of the outlet strut suggested either a design or a manufacturing flaw. The fractures themselves were catastrophic, leading to embolism of the disc, abrupt volume overload, and death in most cases, with significant morbidity reported in many of those who survived to emergent surgery. As a result, although the overall mortality rate for the BSCC was similar to that of other valves implanted during the same period, the 70 ° valve was withdrawn from the market in 1983, and the 60 ° valves were withdrawn in 1986. The fractures of the 70 ° valves were considered to be more common, accounting for its earlier removal from the market.
Mechanism
As the number of fractures began to increase, the Shiley Heart Valve Research Center initiated a number of studies to determine the cause of the outlet strut fractures (OSFs). Pulse duplicator studies demonstrated that (1) uneven pressure distribution across the disc at closure resulted in a tendency for the disc to overrotate, (2) there was a linear relation between outlet strut loads and the closing velocity, (3) outlet strut loads increased with increasing dP/dt and heart rate, and (4) valve-related factors such as the hook-to-well distance and hook deflection correlated with outlet strut load.
Studies of BSCC valves with strain gauges implanted in sheep showed that the overall strut loads increased with increasing exercise associated with increasing dP/dt and pressure at valve closure. Thus, it was concluded that increasing closure forces, most notable during exercise, caused overrotation of the disc that pushed the tip of the outlet strut upward, creating a bending force at the weld site at the base of the strut legs, which at some point exceeded the tolerance of the Haynes 25 alloy. Over time, repeated stresses led to cracking and ultimate fracture of 1 or both outlet struts. This led the manufacturer to increase the hook-to-well distance, and these modified valves were introduced in April 1984. To date, none of these valves has ever fractured, but these data did not become available until long after the BSCC valves were withdrawn from the market.
Activities of the Supervisory Panel
Establishment of guidelines for valve replacement surgery
The Bowling-Pfizer settlement agreement provided monetary benefit for those who had been injured by BSCC failure and for their families, as well as certain costs associated with valve replacement for those those in whom the risk of valve failure exceeded the surgical mortality/serious morbidity involved in valve replacement. The guidelines were therefore intended to identify patients in whom the meaningful extension of life expectancy provided by reoperation exceeded the potential loss due to valve fracture. In calculating this risk, the panel assumed that the patient was in optimal health for his or her age and that the facility where the surgery was to be performed had an excellent operative mortality record. This in turn required assembling data on the incidence of strut fracture, the risk factors associated with fracture, and the surgical mortality and morbidity data for valve reoperation.
Incidence of strut fracture
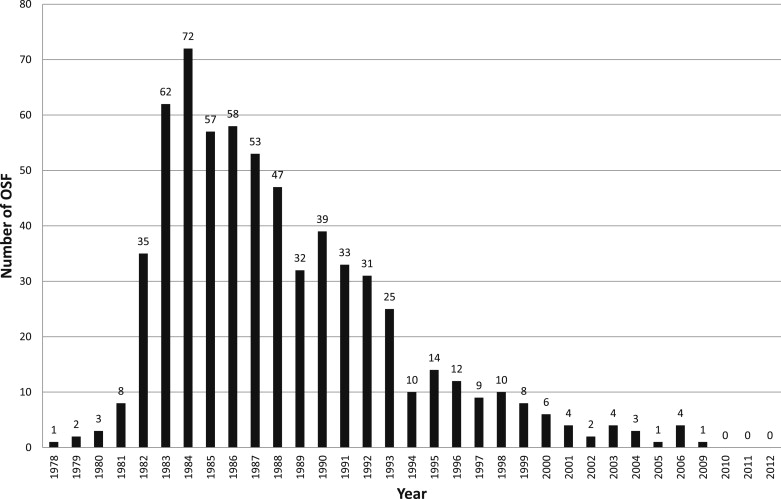
OSFs occurring worldwide have been regularly reported to Pfizer from 1979 to the present. As of the end of 2012, a total of 663 fractures (491 in 60 ° valves and 155 in 70 ° valves) have been reported, including17 reported to Pfizer without serial number identification. The average annual fracture rate over the entire period is 0.04% for 60 ° valves and 0.26% for 70 ° valves, although actual fracture rates are believed to be somewhat higher because not all OSFs are believed to be reported to Pfizer.
Figure 2 shows the numbers of OSFs reported annually from the worldwide database. The number of fractures increased to a peak in the mid-1980s and began a steady decrease thereafter.
Risk factors for OSF
The factors affecting risk of OSF were originally examined in a retrospective cohort study on all 2,303 Dutch patients implanted with 60 ° or 70 ° valves. After an average follow-up period of 6.6 years, the investigators identified 42 cases of OSF. Multivariate analysis identified wide opening angle (70 ° ), large valve size (≥29 mm diameter), and young age (<50 years) as risk factors for OSF. To further explore the determinants of risk and their relative contribution to OSF, the panel sponsored additional case-control and cohort studies in both the US and Europe. In the first of these studies, Walker et al conducted a case-control study of 60 ° valves implanted in the US and Canada and manufactured from January 1, 1979 to March 31, 1984. Cases included all verified OSFs reported to the manufacturer from January 1979 through January 1992. Clinical data were available from medical records for 96 cases and 634 controls. The investigators concluded that “[t]here was a strong inverse gradient of risk with age … Large mitral valves were at greatest risk of strut fracture … valves welded from mid-1981 through March 1984 were more likely to fracture than those manufactured in 1979 and 1980 … Body surface area <1.5 m 2 was associated with 1/16 the risk of body surface area of ≥2.0 m 2 ”. It was noted that differences in body surface area are related to gender, with women having smaller areas.
The panel also sponsored follow-up to the 1992 Dutch study in which the manufacturing characteristics that predicted OSF in large 60 ° degree valves were studied. In this investigation, Kallewaard et al followed all patients with implanted valves until fracture, death, reoperation, or the end of study on July 1, 1996. Manufacturing records were available for 637 valves, including 23 fractured valves. Results indicated that “age at implantation …, lot size …, number of hook deflection tests performed …, number of discs that were used …, and lot fracture percentage … as independent predictors of fractures”. The manufacturing data were provided by Pfizer for this review.
Also sponsored by the panel was a study by Omarand et al who reported on OSF in the United Kingdom cohort of 2,977 patients with a follow-up of 18 years. There were 56 OSFs. The investigators identified age, body surface area, valve size, shop order, fracture rate, and manufacturing period as risk factors for OSF. They also noted that the risk of OSF in valves manufactured from 1981 to 1984 was 4× greater than that of valves manufactured before 1981.
The results of these studies and the calculated relative valve-related risks are summarized in Table 1 . This table has been updated as each new set of fracture data are made available to the panel.
| Risk Factor | Category | Estimated Relative Risk of OSF | Estimated No. (%) of Valves With Attribute | No. (%) of OSF With Attribute |
|---|---|---|---|---|
| Angle | 70 ° | 5.0 | 4,000 (5) | 154 (24) |
| 60 ° | 1.0 ∗ | 81,700 (95) | 479 (76) | |
| Size (mm) | 33 | 9.6 | 1,600 (2) | 58 (10) |
| 31 | 5.5 | 10,300 (12) | 205 (33) | |
| 29 | 4.0 | 14,900 (17) | 181 (28) | |
| 27, 23 | 2.8 | 32,200 (38) | 155 (24) | |
| 25, 21 | 1.0 ∗ | 26,500 (31) | 34 (5) | |
| Weld date | >1980 | 1.0 ∗ | 7,600 (9) | 35 (5) |
| 1980 | 0.5 | 18,400 (22) | 45 (7) | |
| January 1981 to June 1982 | 1.6 | 33,100 (39) | 463 (74) | |
| July 1982 to March 1984 | 1.0 | 18,500 (22) | 89 (14) | |
| April 1984 onward | 0.0 | 8,000 (9) | 0 (0) | |
| Shop order † | OSF in other valves in batch <1% | 1.0 ∗ | 69,800 (81) | 231 (36) |
| OSF in other valves in batch 1%–5% | 1.9 | 12,100 (14) | 247 (39) | |
| OSF in other valves in batch >5% | 2.4 | 3,800 (4) | 155 (24) | |
| Welder group ‡ | A or B | 1.0 ∗ | 70,700 (82) | 391 (62) |
| C | 1.5 | 15,100 (18) | 242 (38) | |
| Position | Mitral | 2.5 | 38,100 (45) | 496 (78) |
| Aorta | 1.0 ∗ | 47,200 (55) | 137 (22) | |
| Rework status § | No crack or rework | 1.0 ∗ | 78,100 (91) | 538 (85) |
| Crack, rework, or missing | 1.6 | 7,700 (9) | 95 (15) |
∗ Reference (baseline) category.
† Batch in which valve was produced.
‡ Refers to categorization of welders who welded the outlet strut to the valve flange.
§ Refers to whether valve was reworked because of a crack or other fault detected during manufacturing process; those with missing information on this factor were also at increased risk and thus grouped with those known to have rework. The risk factors identified for OSF are listed and compared with a reference baseline category of 1. The highest risks occur in 70 ° valves, larger mitral valves, and valves with a crack or that had been reworked.
Risk factors therefore included those related to the valve. Valve opening angle (70 ° vs 60 ° ) and diameter size (33 vs 21 mm) are the strongest determinants of fracture risk. Other significant but weaker determinants were related to specific aspects of the manufacturing process such as the fracture rate of the batch from which the valve originated, the amount of rework of valves, and the welder group.
Age and gender are the most important patient-related characteristics. The risk of fracture was only 1/2 as high among women compared with men, and risk decreased with increasing age. Fewer than 14% of the fractures occurred in those aged ≥65 years ( Table 2 ).
| Age (yrs) | No. of OSF | Incidence Rate (% per yr) | 95% CI |
|---|---|---|---|
| <45 | 30 | 0.29 | 0.19–0.39 |
| 45–54 | 23 | 0.15 | 0.09–0.21 |
| 55–64 | 41 | 0.15 | 0.10–0.19 |
| 65–74 | 13 | 0.04 | 0.02–0.07 |
| 75+ | 2 | 0.02 | 0.00–0.04 |
To determine OSF rates for purposes of the guidelines, we used the periodic reports of OSF data prepared by the manufacturer and submitted to the FDA and the Bowling-Pfizer Trustees ( Table 1 ) as numerators and the estimated valve-years of follow-up as denominators. Unlike the individual studies reported previously, this reporting system includes fractures worldwide. Because not all OSFs are believed to be captured by this system, an upward adjustment of the estimated rates was made by the panel to account for underreporting. The upward adjustment contained the higher observed rates of OSFs in the Netherlands and the United Kingdom cohorts, in which the reporting is considered to be highly accurate. An additional 10% was added to compensate for potential underreporting suggested by these cohorts.
Risk factors to mechanisms of fracture
These epidemiologic data are consistent with the factors known to increase stress on the valve. Specifically, closing stresses increase with increasing cardiac output, heart rate, and valve size and are greatest during periods of intense exercise. All these parameters tend to be greater in men than women and tend to decrease as patients age. They are also consistent with the observation that fracture rate is associated with body mass index. Although one might assume that the cumulative effect of multiple closures over time would increase risk, only those that increased closure forces above the tolerance limit of the strut appear critical.
Risks associated with reoperation
The supervisory panel’s guidelines were heavily dependent on surgical mortality and morbidity data for explantation of prosthetic valves. This was complicated by the fact that data for elective operation for prosthetic valve replacement are limited. Nevertheless, the panel gathered data from the most reliable sources, including the database of the Society of Thoracic and Cardiovascular Surgery and from the Cleveland Clinic, which is the largest center in the world performing heart valve replacement. The surgical mortality and morbidity within 90 days were obtained from the Society of Thoracic and Cardiovascular Surgery database, and early and later-stage mortalities and morbidities were determined from the Cleveland Clinic database. The panel periodically collected data from these sources and corrected its estimates based on improvements in surgical results as it modified the guidelines from time to time. Because the mortality and morbidity for replacement of aortic valves differed from that of the replacement of mitral valves, each was addressed separately.
Because patient age is an important factor in surgical mortality and the average age of the patients with BSCC valves was greater than many of the reports in the literature, the panel needed to make adjustments for these differences. Concomitant disease is also an important contributor to surgical mortality; however, as the panel was required by a binding settlement protocol to assume that the patients were in optimal health and individual patient status was unknown to the panel, risk was based on that for patients in class I and II. It was assumed that the patient’s health would be taken into account by his or her personal physician in deciding on reoperation irrespective of the patient’s qualification for monetary benefits. It is also well known that the surgical mortality and morbidity is much greater at low-volume centers, but the charge to the panel was to determine the optimal surgical mortality and morbidity. Thus, it used the data from the Cleveland Clinic database together with the significant numbers of explants carried out at the Stanford and William Beaumont Hospitals to determine optimal results.
Although the observed operative mortality improved slightly as each of the new guidelines was developed, the decreasing risk of cardiac surgery was counterbalanced by the concomitant aging of the population, with the result that changes that were used in the development of each set of guidelines were relatively small.
In 1997, the court approved the first set of guidelines established by the panel. Since then, the guidelines have been revised 3 times, with the current version being approved in 2007. Because of the relatively small annual fracture rate and the age of the population, the number of patients who were initially expected to achieve an actual improvement in life expectancy was relatively small and consisted mainly of young patients with larger mitral valves. With each revision of the guidelines, this number decreased because of the decreasing fracture rate and the advancing age and decreasing size of the population of patients with BSCC valves.
Issues involved in applying guidelines to individual patients
The first issue relates to the difficulty in applying information obtained from group data to individual patients. The value of the guidelines is in identifying the subgroup of patients for whom, on average, BSCC heart valve reoperation will result in a gain in life expectancy. However, for some individual patients, there can be an immediate loss of life expectancy (if death results from reoperation), whereas for other patients there can be a significant gain (if a strut fracture is avoided by a successful operation). Furthermore, for some patients who undergo reoperation, there may well be no change in life expectancy, even if they survive the reoperation because they may not have had an OSF if the valve had been left in place. Accordingly, it is important to understand that the guidelines are based on a statistical analysis of group data and that the risk for an individual patient may differ from that of the group.
The second issue is that the qualification for a monetary benefit should not be equated with the recommendation that replacement surgery is appropriate for a particular patient. This is because many patients are not in optimal health, and some facilities do not have significant experience in valve replacement surgery. Thus, when either of these assumptions is not met, the risk of surgery would increase and the likelihood of benefit to the patient would decrease.
Finally, although the guidelines define patients who are most likely to benefit from valve replacement surgery, they are based on probabilities, and thus on occasion, patients deemed at low risk may experience fracture. Although the percentage of fractures is considerably less than in the high-risk group, because the low-risk group itself is many-fold larger, the absolute numbers will be greater.
Single leg separation
Experimental studies indicated that a break in a single leg of the outlet strut would occur first, followed after a variable time by complete fracture of the strut, and therefore this phenomenon, termed single leg separation (SLS), was considered a precursor of OSF. Because SLS was considered to place patients with BSCC valves at particularly high risk, monetary benefits were included in the settlement to cover certain costs associated with explant surgery for these patients. This led to a number of research studies to develop methods to detect SLS in patients with BSCC valves.
Over time, individual prophylactically explanted valves returned to the manufacturer after explantation showed evidence of SLS by visual inspection or microscopic examination. The prevalence of SLS in these valves was 8.2%, but this varied by valve size, position, and opening angle. The size-, position-, and angle-specific SLS rates were applied to the corresponding valve distribution in the worldwide database. This resulted in an estimated SLS prevalence of 6.8% (95% confidence limits 4.1% to 9.4%) in all valves.
Activities of the Supervisory Panel
Establishment of guidelines for valve replacement surgery
The Bowling-Pfizer settlement agreement provided monetary benefit for those who had been injured by BSCC failure and for their families, as well as certain costs associated with valve replacement for those those in whom the risk of valve failure exceeded the surgical mortality/serious morbidity involved in valve replacement. The guidelines were therefore intended to identify patients in whom the meaningful extension of life expectancy provided by reoperation exceeded the potential loss due to valve fracture. In calculating this risk, the panel assumed that the patient was in optimal health for his or her age and that the facility where the surgery was to be performed had an excellent operative mortality record. This in turn required assembling data on the incidence of strut fracture, the risk factors associated with fracture, and the surgical mortality and morbidity data for valve reoperation.
Incidence of strut fracture
OSFs occurring worldwide have been regularly reported to Pfizer from 1979 to the present. As of the end of 2012, a total of 663 fractures (491 in 60 ° valves and 155 in 70 ° valves) have been reported, including17 reported to Pfizer without serial number identification. The average annual fracture rate over the entire period is 0.04% for 60 ° valves and 0.26% for 70 ° valves, although actual fracture rates are believed to be somewhat higher because not all OSFs are believed to be reported to Pfizer.
Figure 2 shows the numbers of OSFs reported annually from the worldwide database. The number of fractures increased to a peak in the mid-1980s and began a steady decrease thereafter.




