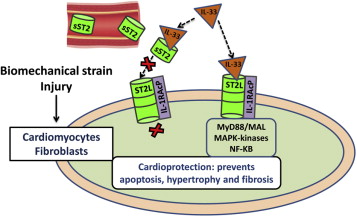
ST2 is a member of the interleukin 1 receptor family with 2 main isoforms: transmembrane or cellular (ST2L) and soluble or circulating (sST2) forms. ST2 is the receptor of the IL-33, which is an IL-1–like cytokine that can be secreted by living cells in response to cell damage. IL-33 exerts its cellular functions by binding a receptor complex composed of ST2L and IL-1R accessory protein. The IL-33/ST2 system is upregulated in cardiomyocytes and fibroblasts as response to mechanical stimulation or injury. The interaction between IL33 and ST2L has been demonstrated to be cardioprotective: in experimental models, this interaction reduces myocardial fibrosis, prevents cardiomyocyte hypertrophy, reduces apoptosis, and improves myocardial function. The beneficial effects of IL-33 are specifically through the ST2L receptor. sST2 avidly binds IL-33 which results in interruption of the interaction between IL-33/ST2L and consequently eliminates the antiremodeling effects; thus, sST2 is viewed as a decoy receptor. In recent years, knowledge about ST2 role in the pathophysiology of cardiovascular diseases has broadly expanded, with strong links to myocardial dysfunction, fibrosis, and remodeling. Beyond its myocardial role, the IL-33/ST2 system could have an additional role in the development and progression of atherosclerosis. In conclusion, IL-33/ST2L signaling is a mechanically activated, cardioprotective fibroblast–cardiomyocyte paracrine system, which may have therapeutic potential for beneficially regulating the myocardial response to overload and injury. In contrast, sST2 acts as a decoy receptor and, by sequestering IL-33, antagonizes the cardioprotective effects of IL-33/ST2L interaction.
ST2 is a member of the interleukin 1 receptor family and is formally known as interleukin 1 receptor–like 1 (IL1RL-1); ST2 was first described in 1989, but it remained for years as an orphan receptor mainly related with immune and inflammatory diseases. In 2002, Weinberg et al reported ST2 expression in cardiac cells as a response to myocardial stress, identifying a role in the cardiovascular system. In 2005, Schmitz et al identified interleukin 33 (IL-33) as the ligand of ST2, which has allowed to improve the understanding of their functions. In the last years, knowledge about ST2 biology and its role in the pathophysiology of cardiovascular diseases has broadly expanded. In the following paragraphs, we present the key issues on ST2 biology, to facilitate the understanding of its role in cardiovascular diseases.
ST2 Isoforms and Regulation
The gene named ST2 is placed on human chromosome 2q12 and is part of the larger IL-1 gene cluster (GenBank accession number AC007248). Four isoforms are the transcriptional product of the gene and 2 of them are the most important: a transmembrane receptor (ST2L or IL1RL1-b) and a truncated soluble receptor that can be detected circulating in serum (sST2 or IL1RL1-a; Figure 1 ). Alternative promoter splicing and 3′ processing of the same mRNA seem to be responsible for the production of sST2 and ST2L. The ST2 gene shows 2 promoters: a proximal one and a distal one, which are able to influence the mechanism of transcriptional regulation of the gene. Each promoter influences the expression of sST2 and ST2L mRNA: the alternative splicing involving the 3′ end of the gene is responsible for this difference ( Figure 1 ). Data about the inner control of differential transcription of sST2 and ST2L are poorly known and restricted to the hematopoietic cells (GATA2, neurotrophin p75 receptor). Importantly, genetic factors appear account for up to 40% of interindividual variability in sST2 levels.
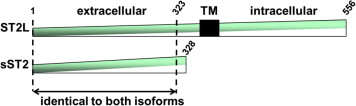
The overall structure of ST2L is similar to the structure of the type I IL-1 receptors: it is a membrane-bound form containing an extracellular domain of 3 linked immunoglobulin-like motifs, a transmembrane segment, and an intracellular Toll/interleukin-1 receptor cytoplasmic domain. As noted, sST2 is a circulating form, which lacks the transmembrane and cytoplasmic domains and includes a unique 9 amino acid C -terminal sequence. The transmembrane form ST2L is constitutively expressed primarily in hematopoietic cells (Th2 and mast cells). The expression of the circulating form sST2 is largely inducible and almost ubiquitous in living cells.
ST2 and IL-33 Interaction
In 2005, IL-33 (also known as IL-1F11) was identified as the ligand of ST2. IL-33 is an IL-1–like cytokine that can be secreted by most cells in response to damage. IL-33 exerts its cellular functions by binding a receptor complex composed of ST2L and IL-1R accessory protein (IL-1RAcP). IL-1RAcP is essential for IL-33 signaling through ST2L by enhancing the affinity of IL-33 for ST2L. The interaction of IL-33 and ST2L activates mitogen-activated protein kinases and several biochemical pathways. The sum total of these events is the activation of the inhibitor of nuclear factor-κB (NF-κB) kinase complex, triggering NF-κB activity. It has been also suggested that IL-33 might have intracellular functions that are independent of binding to the ST2L receptor.
sST2 avidly binds IL-33 which results in interruption of the interaction between IL-33/ST2L, with consequent abrogation of their cellular functions; sST2 is thus thought to be a decoy receptor ( Figure 2 ). In this manner, the ST2 system acts not only as a mediator of IL-33 function in its ST2L transmembrane isoform but also as an inhibitor of IL-33 through its soluble sST2 isoform. In addition, IL-33 appears to also regulate ST2L and sST2 mRNA transcription, increasing the mRNA expression of former while reducing the latter. Therefore, there is a reciprocal influence between cytokines and ST2.
ST2 and IL-33 Interaction
In 2005, IL-33 (also known as IL-1F11) was identified as the ligand of ST2. IL-33 is an IL-1–like cytokine that can be secreted by most cells in response to damage. IL-33 exerts its cellular functions by binding a receptor complex composed of ST2L and IL-1R accessory protein (IL-1RAcP). IL-1RAcP is essential for IL-33 signaling through ST2L by enhancing the affinity of IL-33 for ST2L. The interaction of IL-33 and ST2L activates mitogen-activated protein kinases and several biochemical pathways. The sum total of these events is the activation of the inhibitor of nuclear factor-κB (NF-κB) kinase complex, triggering NF-κB activity. It has been also suggested that IL-33 might have intracellular functions that are independent of binding to the ST2L receptor.
sST2 avidly binds IL-33 which results in interruption of the interaction between IL-33/ST2L, with consequent abrogation of their cellular functions; sST2 is thus thought to be a decoy receptor ( Figure 2 ). In this manner, the ST2 system acts not only as a mediator of IL-33 function in its ST2L transmembrane isoform but also as an inhibitor of IL-33 through its soluble sST2 isoform. In addition, IL-33 appears to also regulate ST2L and sST2 mRNA transcription, increasing the mRNA expression of former while reducing the latter. Therefore, there is a reciprocal influence between cytokines and ST2.
ST2 in Inflammatory Diseases
Before recognition of a cardiovascular role for ST2, most knowledge about the marker had been related to inflammatory and immune processes, particularly regarding the regulation of mast cells and type 2 CD4 + T-helper cells, and the production of Th2-associated cytokines. IL-33/ST2 signaling participates in the immune response through the activation of Th2 effector cells and the release of Th2-related cytokines. Therefore, a role for IL-33/ST2 has been demonstrated in numerous diseases associated with a predominant Th2 response such as asthma, pulmonary fibrosis, rheumatoid arthritis, collagen vascular diseases, sepsis, trauma, malignancy, fibroproliferative diseases, helminthic infection, and ulcerative colitis. ST2L mediates the effect of IL-33 on Th2-dependent inflammatory processes, whereas sST2 has been implicated in the attenuation of these Th2 inflammatory responses.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


