Chapter 1 The atmosphere
 The mass of the Earth and its distance from the sun provide optimal conditions of gravity and temperature for long-term liquid surface water and the retention in its atmosphere of oxygen, nitrogen and carbon dioxide.
The mass of the Earth and its distance from the sun provide optimal conditions of gravity and temperature for long-term liquid surface water and the retention in its atmosphere of oxygen, nitrogen and carbon dioxide. Primitive life-forms generated energy by photosynthetic reactions, producing oxygen, and so facilitating the development of an oxygen-containing atmosphere and aerobic organisms.
Primitive life-forms generated energy by photosynthetic reactions, producing oxygen, and so facilitating the development of an oxygen-containing atmosphere and aerobic organisms. Carbon dioxide was initially the main component of the Earth’s atmosphere, but by 300 million years ago rock weathering and photosynthesis had reduced its concentration to current low levels.
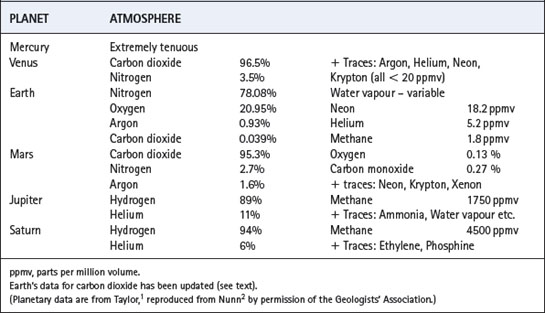
Carbon dioxide was initially the main component of the Earth’s atmosphere, but by 300 million years ago rock weathering and photosynthesis had reduced its concentration to current low levels.The atmosphere of Earth is radically different from that of any other planet in the solar system (Table 1.1) and may well be rare on planets of other stars in the universe as a whole. The unique character of our atmosphere is because of two main reasons. First, temperature has permitted the existence of liquid surface water for at least 3800 million years (Ma), and this has resulted in weathering of silicate rocks, reducing the concentration of carbon dioxide far below the levels still pertaining in the rocky planets Venus and Mars. Secondly, the existence of liquid surface water enabled living organisms to appear at a very early stage: life forms then evolved to undertake oxygenic photosynthesis. When oxygen sinks were saturated, oxygen appeared in the atmosphere and some organisms began to utilise highly efficient oxidative metabolic pathways. An atmosphere containing oxygen is in inorganic chemical disequilibrium, and is an indication of the existence of life.
Evolution of the Atmosphere
Formation of the Earth and the Pre-Biotic Atmosphere
Earth cooled rapidly by radiation when the initial bombardment abated, and the very high temperature (Hadean) phase is not thought to have lasted longer than a few hundred Ma. The crust solidified, but massive outgassing continued, resulting in an atmosphere mainly comprising carbon dioxide and steam (Table 1.2) as probably occurred on Venus and Mars.1,2 In the case of Earth, the water vapour condensed to surface water, and there is good evidence that oceans existed about 3800 Ma ago and perhaps even earlier.3 Once Earth’s crust was cool, and surface water was in existence, it was possible for comets and meteorites to leave a secondary veneer of their contents, including water and a wide range of organic compounds.4
Table 1.2 Average composition of gas evolved from Hawaiian volcanoes
| CONSTITUENT | PERCENT |
|---|---|
| Water vapour | 70.75 |
| Carbon dioxide | 14.07 |
| Sulphur dioxide | 6.40 |
| Nitrogen | 5.45 |
| Sulphur trioxide | 1.92 |
| Carbon monoxide | 0.40 |
| Hydrogen | 0.33 |
| Argon | 0.18 |
| Sulphur | 0.10 |
| Chlorine | 0.05 |
(Data are from reference 5, reproduced from reference 2 by permission of the Geologists’ Association.)
Important physico-chemical changes occurred in the early secondary atmosphere. Helium and hydrogen tended to be lost from the Earth’s gravitational field. Ammonia dissociated to nitrogen and hydrogen, the former retained and the latter lost from the atmosphere. Some carbon dioxide might have been reduced by hydrogen to form traces of methane, but very large quantities slowly reacted with surface silicates to become trapped as carbonates, while forming silica (weathering). Traces of water vapour underwent photodissociation to hydrogen and oxygen. However, oxygen from this source was present in only minimal quantities, and the early atmosphere is no longer thought to have been as strongly reducing as was formerly believed.6
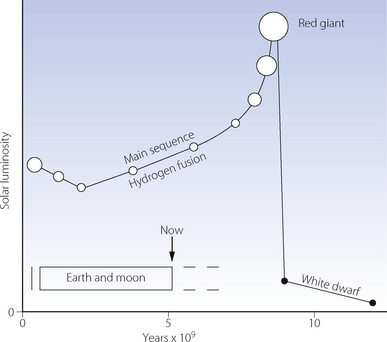
The initial very high partial pressure of carbon dioxide, and probably some methane, would have provided a powerful greenhouse effect to offset the early minimal weak solar radiation, which was some 30% less than today (Figure 1.1). However, the Sun commenced its main sequence of thermo-nuclear fusion of hydrogen to helium about 3000 Ma ago. Since then solar radiation has been increasing steadily as the Sun proceeds remorselessly towards becoming a red giant, which will ultimately envelop the inner planets. It is fortunate that increasing solar radiation has been approximately offset by a diminishing greenhouse effect, due mainly to decreasing levels of carbon dioxide (see below). As a result, Earth’s temperature has remained relatively stable, permitting the existence of surface water for the last 3800 Ma.
Significance of Mass of Earth and Distance from Sun
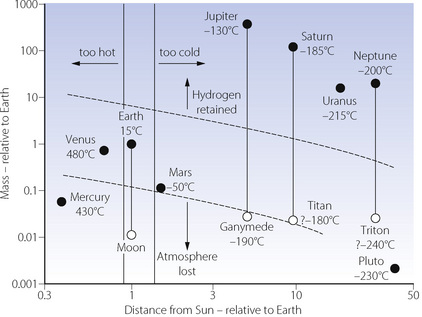
Small bodies, such as Mercury and most of the planets’ satellites, have a gravitational field which is too weak for the retention of any significant atmosphere (Figure 1.2). The gas-giants (Jupiter, Saturn, Uranus and Neptune) have a gravitational field which is sufficiently strong to retain all gases, including helium and hydrogen, thereby ensuring the retention of a reducing atmosphere. The gravitational field of the Earth is intermediate, resulting in a differential retention of the heavier gases (oxygen, carbon dioxide and nitrogen), while permitting the escape of hydrogen and helium. This is essential for the development of an oxidising atmosphere and life as we know it. Water vapour (molecular weight only 18) would be lost from the atmosphere were it not for the cold trap at the tropopause.
Surface temperature of a planetary body is crucial for the existence of liquid water, which is essential for life and therefore the composition of our atmosphere. To a first approximation, temperature is dependent on the distance of a planet from the Sun, and the intensity of solar radiation (Figure 1.2). The major secondary factor is the greenhouse effect of any atmosphere which the planet may possess. Mercury and Venus have surface temperatures far above the boiling point of water. All planets (and their satellites) which are further away from the Sun than Earth have a surface temperature too cold for liquid water to exist today. However, there is now evidence that Mars had liquid surface water in the past,8 now present only as ice.9
Earth is the only planet in the solar system which has both a mass permitting retention of an oxidising atmosphere, and a distance from the Sun at which the temperature permits liquid water to exist on its surface. It is difficult to see how there could be life as we know it anywhere in the solar system outside the small parallelogram in Figure 1.2. However, an environment similar to that of the earth may well exist on some planets of the 1022 other sun-like stars in the universe.
Origin of Life and the Development of Photosynthesis
Amino acids and a wide range of organic compounds are found in a type of meteorite known as carbonaceous chondrites.4 Therefore, whether or not such compounds were actually synthesised on the early Earth, as Stanley Miller had proposed,6 it is highly likely that a wide range of organic compounds were available on the pre-biotic Earth when liquid oceans were formed.
It is less easy to explain the next stage in the evolution of life. An essential feature of all life is the synthesis of proteins using a ribonucleic acid (RNA) template, usually transcribed from the genetic code carried on deoxyribonucleic acid (DNA). There would appear to have been a classical ‘chicken and egg’ situation. Useful proteins could not be formed without the appropriate sequences in RNA or DNA: RNA and DNA could not be polymerised without appropriate enzymes which are normally proteins. Nevertheless, life did appear, perhaps in the first instance with the genetic code carried only on RNA, or even the much simpler peptide nucleic acid (PNA).10
An essential requirement for life is the availability of bio-usable forms of energy. The forms of available energy and their location at the dawn of life remain a mystery. However, one cannot ignore the possibility of hydrothermal vents, such as the black smokers along the mid-ocean ridges at great depths, which still support very simple life forms on the basis of chemoautotrophy. They are totally independent of sunlight, and exploit the profound chemical disequilibrium between the emerging hot, reducing and acid water, containing hydrogen sulphide, methane, ammonia, phosphorus and a range of metals, and the surrounding sea water.11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree