There is wide variation in the management of patients with atrial fibrillation (AF) in the emergency department (ED). We aimed to derive and internally validate the first prospective, ED-based clinical decision aid to identify patients with AF at low risk for 30-day adverse events. We performed a prospective cohort study at a university-affiliated tertiary-care ED. Patients were enrolled from June 9, 2010, to February 28, 2013, and followed for 30 days. We enrolled a convenience sample of patients in ED presenting with symptomatic AF. Candidate predictors were based on ED data available in the first 2 hours. The decision aid was derived using model approximation (preconditioning) followed by strong bootstrap internal validation. We used an ordinal outcome hierarchy defined as the incidence of the most severe adverse event within 30 days of the ED evaluation. Of 497 patients enrolled, stroke and AF-related death occurred in 13 (3%) and 4 (<1%) patients, respectively. The decision aid included the following: age, triage vitals (systolic blood pressure, temperature, respiratory rate, oxygen saturation, supplemental oxygen requirement), medical history (heart failure, home sotalol use, previous percutaneous coronary intervention, electrical cardioversion, cardiac ablation, frequency of AF symptoms), and ED data (2 hours heart rate, chest radiograph results, hemoglobin, creatinine, and brain natriuretic peptide). The decision aid’s c-statistic in predicting any 30-day adverse event was 0.7 (95% confidence interval 0.65, 0.76). In conclusion, in patients with AF in the ED, Atrial Fibrillation and Flutter Outcome Risk Determination provides the first evidence-based decision aid for identifying patients who are at low risk for 30-day adverse events and candidates for safe discharge.
Atrial fibrillation (AF) affects an estimated 33.5 million subjects worldwide and is associated with a 2-fold increased mortality. The management of AF accounts for an estimated $26 billion in annual health care costs in the United States. Hospitalizations constitute the majority of the AF health care expenses. Compared with matched controls, patients with AF reported >2-fold emergency department (ED) evaluations and hospitalizations. The ED is the gatekeeper for acute AF management as >70% of patients hospitalized for AF are initially managed there. Between 25% and 35% of these visits are for new AF diagnoses. Hospitalizations after an ED evaluation for AF vary considerably with admission frequencies of 81%, 62%, and 24% in the United States, Australia, and Canada, respectively. The lack of accurate risk stratification may contribute to substantial variation in ED dispositions. Although AF increases a patient’s lifetime risk of death and stroke, the 30-day risk of stroke and death after an ED visit for primary AF is relatively low with a combined incidence of 1% to 3%. The combination of a high hospitalization rate and low incidence of 30-day serious adverse events provides an excellent opportunity to deliver more standardized and resource-efficient treatment. Decision aids are an important component of ED practice resulting in improved patient care of common presenting complaints. We previously reported the first decision aid for estimating 30-day adverse event risk in a retrospective cohort of patients with AF in the ED. Our objective was to derive the first prospective AF decision aid that incorporated data available within the initial 2 hours of ED evaluation and accurately predicted risk for 30-day adverse events.
Methods
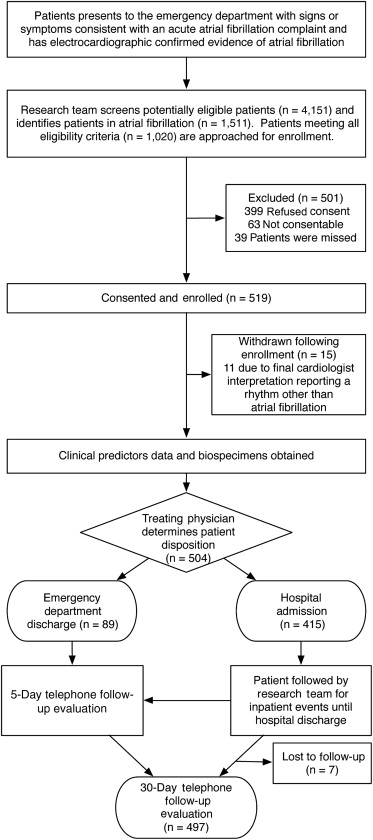
We conducted a prospective observational cohort study at Vanderbilt University Medical Center’s ED from June 9, 2010, to February 28, 2013. The adult ED is a university-affiliated, urban, tertiary care, referral center with an annual patient census of 70,000. The details of the Atrial Fibrillation and Flutter Outcome Risk Determination (AFFORD) study design have been previously reported. Briefly, the study team consisting of the principal physician investigator and trained clinical trial associates screened patients in ED for potential eligibility ( Figure 1 ). We enrolled a convenience sample of patients presenting with signs (e.g., tachycardia, dyspnea) or symptoms (e.g., palpitations, chest pain, shortness of breath, weakness, presyncope, or syncope) consistent with symptomatic AF that prompted the treating emergency physician to obtain an electrocardiogram. The diagnosis of AF or atrial flutter was based on the emergency physician’s interpretation of the electrocardiogram. All electrocardiogram interpretations were confirmed by an independent cardiologist review of the electrocardiogram within 24 hours of enrollment. Patients whose electrocardiograms were determined to be a rhythm other than AF, based on the cardiologist’s interpretation, were withdrawn from the study. Patients with acute life-threatening conditions (i.e., stroke, myocardial infarction, sepsis) were not enrolled. Treatment and disposition decisions for patients enrolled in the study were determined by the treating physicians and were not influenced by this investigation. Our medical center’s institutional review board reviewed and approved this study. Patients provided written informed consent.
We included both patients whose ED evaluations were primarily for AF and patients with AF presenting with an alternative primary disease. To determine whether the evaluation was primarily for AF or whether AF was complicating an alternative acute process, a board-certified emergency medicine physician investigator reviewed the ED records of all enrolled patients. The principal investigator used a standardized protocol. A board-certified electrophysiologist investigator provided a second review of a randomly selected 31% subset of the enrollments. We measured the absolute agreement and Cohen’s kappa statistic with 95% confidence intervals (CIs) between the 2 reviewers’ designation for the primary cause of ED visits.
Clinical trial associates collected data on consented subjects by direct questioning and a review of the electronic health record. Study data were collected and managed using Research Electronic Data Capture. The principal investigator reviewed and confirmed the accuracy of the data recorded by the clinical trial associates. To minimize missing data, we performed laboratory testing using frozen, stored blood specimens when such tests were not done as standard ED treatment. We defined AF type in accordance with international guidelines.
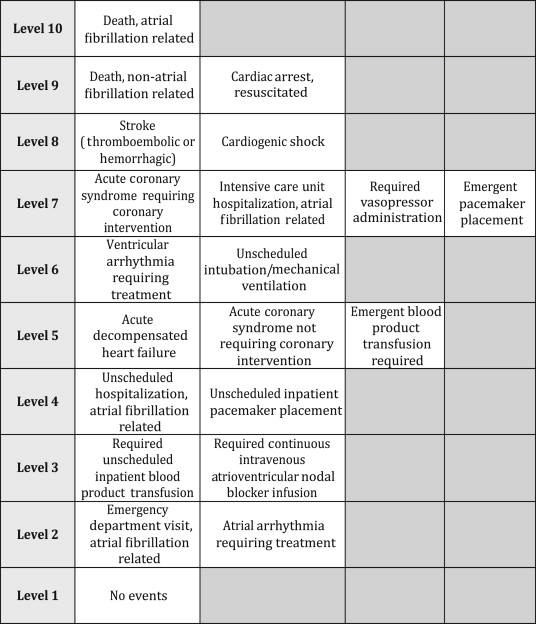
The primary outcome was a 10-level ordinal outcome representing the most severe adverse event experienced within 30 days of the index ED evaluation ( Figure 2 ). The events of the outcome were ordered from least severe (no event) to most severe (AF related). The hierarchy was determined by consensus among the investigators including 3 emergency physicians and 3 electrophysiologists. We reviewed the patient’s records and the Social Security Death Index for outcomes. Admitted patients were followed until hospital discharge. All patients received structured follow-up telephone communication at 5 and 30 days from the ED visit. Investigators used a standard telephone communication data collection form for all interviews. The investigators assessing the presence of each outcome event were masked to the predictor variables and vice versa. The accurate determination of whether the adverse events were related to AF was of utmost importance for this study. The principal investigator and an electrophysiologist co-investigator used a standardized protocol to determine whether an adverse event was AF related.
A large pool of candidate predictor variables were considered based on established risk factors for AF, focusing on those likely to be recorded in the ED and chosen in accordance with established principles. Development of the AFFORD decision aid followed established strategies that are detailed in Supplementary Material . Briefly, model selection from the pool of candidate predictors was done using a model approximation method called preconditioning. Preconditioning is suited for high dimensional data problems in which the number of predictors is large relative to the number of events. Proportional odds logistic regression was then used to fit the ordinal outcome against the selected predictors. An ordinal outcome was used rather than a binary outcome because the proportional odds model allows for parsimonious modeling of an ordinal outcome with increased power and precision compared with a binary logistic model. The AFFORD decision aids was then obtained by assigning points for selected predictors based on their corresponding regression coefficients.
We quantified AFFORD’s predictive accuracy in predicting any 30-day adverse event using a concordance index (c-statistic). The calibration was assessed using a smooth nonparametric calibration curve comparing predicted and observed probabilities of any 30-day adverse event for the original model (apparent) and the bootstrapped bias-corrected model (bias corrected). In general, the calibration curve illustrates bias in the predicted values obtained from the prediction model. We performed a strong internal validation using bootstrap resampling to estimate the likely performance of the decision aid on a new sample of patients from the same patient stream. All analyses were done using R programming language (R Foundation for Statistical Computing, Vienna, Austria). Standard sample size requirements, supported by simulation studies and expert opinion, include 15 subjects or events per degree of freedom (i.e., per regression coefficient examined or estimated). Our initial planned sample size of 430 patients accounted for the a priori determined predictors and an anticipated 5% loss to follow-up. We exceeded the planned sample size because of higher-than-expected ED volumes of eligible patients with AF. The entire sample was used in model development.
Results
This study enrolled 519 patients from June 9, 2010, to February 28, 2013, with 15 withdrawn and 7 lost to follow-up ( Figure 1 ). The 15 withdrawals were primarily for failure to meet inclusion criteria when the ED electrocardiogram was subsequently interpreted as a rhythm other than AF or flutter by the independent cardiologist. A search of the Social Security death index did not find any reported deaths among these withdrawals within 30 days of their ED visit. Of the 4,151 patients screened, only 39 (<1%) subjects were classified “missed potentially eligible.”
Table 1 reports the baseline characteristics. The characteristics of the 501 potentially eligible patients who refused to participate (n = 399) or were missed were similar to enrolled patients. Of the 497 patients in the cohort, the principal investigator determined that 326 (65.6%) were for primary AF. An electrophysiologist co-investigator independently reviewed 163 (31.4%) records to determine whether AF was the primary reason for the ED visit. Of those 163 records, concordance was found in 135 (83%), corresponding to a kappa of 0.8 (95% CI 0.7 to 0.9).
| Characteristic | Study Population (n=497) ∗ |
|---|---|
| Age (years) | 68 (58, 78) |
| Female | 177 (36%) |
| White | 447 (90%) |
| Black | 50 (10%) |
| Insurance status: Medicare | 220 (44%) |
| Private/Group | 244 (49%) |
| Federal (Medicaid/Veterans Administration) | 12 (2%) |
| Self-pay | 21 (4%) |
| ED visit For Primary Atrial Fibrillation | 326 (66%) |
| Type of Atrial Fibrillation | |
| New diagnosis | 131 (26%) |
| Paroxysmal | 183 (37%) |
| Persistent | 61 (12%) |
| Permanent | 122 (25%) |
| CHA 2 DS 2 -VASc | 3 (2, 5) |
| Complaint in the Emergency Department | |
| Chest pain | 90 (18%) |
| Palpitations | 44 (9%) |
| Shortness of breath | 78 (16%) |
| Fatigue | 332 (67%) |
| Triage blood pressure, systolic (mm Hg) | 134 (117, 152) |
| Triage blood pressure, diastolic (mm Hg) | 80 (69, 92) |
| Triage heart rate (beats per minute) | 105 (82, 130) |
| Triage respiratory rate | 18 (16, 20) |
| Triage oxygen saturation (%) | 97 (95, 98) |
| ED supplemental oxygen requirement | 58 (12%) |
| Duration of symptoms prior to ED presentation (hours) | |
| < 12 | 213 (43%) |
| 12-24 | 62 (12%) |
| 24-36 | 24 (5%) |
| 36-48 | 18 (4%) |
| >48 | 123 (25%) |
| Unknown | 56 (11%) |
| Maximum pulse rate during initial 2 hours of ED management (beats per minute) | 123 (91, 144) |
| Heart rate at 2 hours following placement in ED room | 93 (77, 116) |
| 2-hour rhythm strip interpretation | |
| Atrial fibrillation | 309 (62%) |
| Atrial flutter | 81 (16%) |
| Sinus rhythm | 58 (12%) |
| Other | 12 (2%) |
| Home aspirin use | 226 (45%) |
| Home beta-blocker use | 259 (52%) |
| Home diltiazem/verapamil use | 86 (17%) |
| Home sotalol use | 36 (7%) |
| Home warfarin use | 166 (33%) |
| Therapeutic INR (2-3.4) † | 108 (65%) |
| Home statin use | 223 (45%) |
| Home ACEI/ARB use | 227 (46%) |
| Home clopidogrel use | 52 (10%) |
| Home novel anticoagulant use | 16 (3%) |
| Home thyroid replacement | 79 (16%) |
| Current smoker | 60 (12%) |
| Current alcohol drinker | 132 (28%) |
| Prior cocaine use | 38 (7%) |
| Prior coronary artery disease | 169 (34%) |
| Prior COPD | 78 (16%) |
| Prior hypertension | 356 (72%) |
| Prior valvular heart disease | 132 (27%) |
| Aortic Regurgitation | 29 (6%) |
| Aortic Stenosis | 24 (5%) |
| Mitral Regurgitation | 91 (18%) |
| Mitral Stenosis | 12 (2%) |
| Pulmonic Regurgitation | 10 (2%) |
| Pulmonic Stenosis | 1 (0.2%) |
| Tricuspid Regurgitation | 49 (10%) |
| Tricuspid Stenosis | 1 (0.2%) |
| Prior cerebrovascular accident | 67 (13%) |
| Prior transient ischemic attack | 62 (12%) |
| Prior heart failure | 167 (34%) |
| Prior renal insufficiency | 88 (18%) |
| Prior non-insulin dependent diabetes | 66 (13%) |
| Prior insulin-dependent diabetes | 62 (12%) |
| Permanent pacemaker | 80 (16%) |
| Family history of atrial fibrillation | 95 (19%) |
| Prior percutaneous coronary intervention | 82 (16%) |
| Prior electrical cardioversion | 19 (4%) |
| Prior cardiac ablation | 44 (9%) |
| Frequency of irregular heartbeat: | |
| Never | 188 (38%) |
| Sometimes | 92 (19%) |
| Usually | 57 (11%) |
| Always | 131 (26%) |
| Hemoglobin (g/dL) | 14 (12, 15) |
| Blood urea nitrogen (mg/dL) | 17 (13, 24) |
| Creatinine (mg/dL) | 1.07 (0.86, 1.39) |
| Troponin I (ng/mL) | 0.02 (0.01, 0.03) |
| Brain natriuretic peptide (pg/mL) | 261 (114, 538) |
| ED disposition, admit | 415 (83.5%) |
| Hospital length of stay (days) | 2.4 (1.0, 4.4) |
∗ N equal total number of non-missing responses for each variable. Categorical variables presented as number followed by percentage in parentheses. Continuous variables are represented as the median with interquartile range in parentheses.
† The reported percentage is of the 166 patients who reported that they were taking warfarin at the time of ED evaluation.
Of the 82 patients who were discharged home, 73 (89%) had no 30-day adverse events, 6 returned to the ED for an AF reason but were not hospitalized and 3 required an unscheduled AF-related hospitalization. Among the 415 patients who were hospitalized after their index ED evaluation, 291 (70%) experienced no 30-day adverse events and 22 had an AF-related return ED evaluation but were not hospitalized. Table 2 reports the incidence of 30-day adverse events.
| 30-Day Adverse Events | No. (%) (n = 497) | AFFORD Hierarchy Level |
|---|---|---|
| Death, AF-related | 4 (<1%) | 10 |
| Death, all cause | 30 (6%) | 9-10 |
| Cardiac arrest, resuscitated | 13 (3%) | 9 |
| Stroke (thromboembolic or hemorrhagic) | 13 (3%) | 8 |
| Cardiogenic shock | 4 (<1%) | 8 |
| Acute coronary syndrome requiring emergent PCI | 0 | 7 |
| Intensive care unit admission, AF-related | 12 (2%) | 7 |
| Vasopressor administration required | 11 (2%) | 7 |
| Emergent pacemaker placement | 1 (<1%) | 7 |
| Ventricular arrhythmia requiring emergent treatment | 7 (1%) | 6 |
| Unscheduled intubation/mechanical ventilation | 12 (2%) | 6 |
| Acute decompensated heart failure | 13 (3%) | 5 |
| Acute coronary syndrome not requiring emergent PCI | 7 (1%) | 5 |
| Emergent blood product transfusion required | 8 (2%) | 5 |
| Unscheduled hospitalization, AF-related | 32 (6%) | 4 |
| Unscheduled inpatient pacemaker placement | 6 (1%) | 4 |
| Unscheduled inpatient blood product transfusion required | 13 (3%) | 3 |
| Continuous AV nodal blocker infusion required | 17 (3%) | 3 |
| ED visit, AF-related | 39 (8%) | 2 |
| Atrial arrhythmia requiring emergent treatment ∗ | 73 (15%) | 2 |
| Experienced ≥1 adverse event | 133 (27%) | 2-10 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


