TEVAR for Acute and Chronic Aortic Dissection
Joseph Lombardi
Carlos A. Neves
Aortic dissection occurs when an intimal tear in the aortic wall is present. This leads to the formation of a false lumen with concomitant delamination between the layers of the intima and media (Table 30.1). This is a potentially life-threatening condition and patients usually present with an acute onset of severe, sharp chest pain that radiates to the neck or back. Some other common features include paresthesias and severe limb pain in the setting of acute arterial ischemia from malperfusion. A pulse deficit in a patient who presents with chest and/or back pain should always raise the concern for an acute aortic dissection. There are a number of factors that seem to play a role in a patient’s immediate survival and long-term outcome, with the biggest challenge being early recognition. Most patients who suffer serious complications of malperfusion or rupture are in a very time-sensitive situation and success to treatment is dependent on prompt diagnosis.
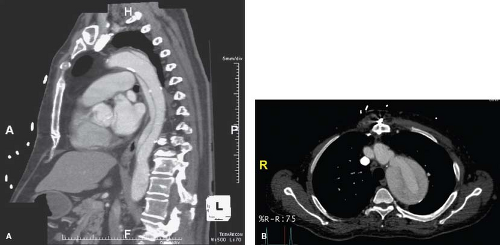
Aortic dissections are a more common disease process in patients in their sixth to seventh decades of life with a greater preponderance for men (4:1). They are classified based on the location of the tears as well as time of diagnosis. Dissections with entry tear locations proximal to the left subclavian artery is classified as type A, and those distal to the left subclavian are type B. Acute dissections (Fig. 30.1A) are diagnosed within 14 days after symptoms first appear, while chronic dissections (Fig. 30.1B) appear after the 14-day mark. For the scope of this chapter we will be describing the endovascular treatment of complicated Type B aortic dissections (cTBAD) that has rapidly become the treatment of choice.
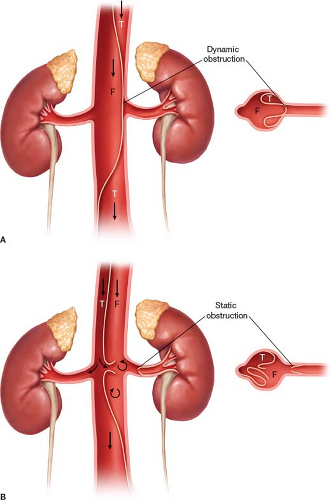
Malperfusion can occur following aortic dissection and results in an inadequate amount of blood supply to vital organs secondary to aortic branch vessel obstruction. This leads to end-organ ischemia and increases the morbidity and mortality associated with aortic dissection. Malperfusion can be divided into dynamic or static. Dynamic malperfusion occurs when a dissection flap intermittently obstructs the vessel origin leading to malperfusion of the end organ (Fig. 30.2A). Meanwhile static malperfusion occurs when the dissection flap directly occludes or narrows the vessel orifice (Fig. 30.2B). Patients that fail medical management or those with static or dynamic malperfusion usually require surgical intervention to relieve the obstruction. Although
medical management has been the hallmark of treatment for uncomplicated Type B dissections, thoracic endovascular aortic repair (TEVAR) has been quickly adopted as the treatment of choice for cTBADs.
medical management has been the hallmark of treatment for uncomplicated Type B dissections, thoracic endovascular aortic repair (TEVAR) has been quickly adopted as the treatment of choice for cTBADs.
Table 30.1 Indications for TEVAR for Treatment of cTBADs | ||||||
|---|---|---|---|---|---|---|
|
Since the introduction of the modern endovascular devices, there has been a change from an open approach to a minimally invasive endovascular one. The goal of TEVAR in this setting is to cover the primary entry tears and promote true lumen flow. Below is a list of indications for TEVAR in the management of cTBADs.
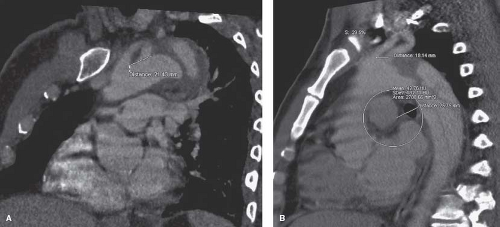
Contraindications to performing a TEVAR for aortic dissections can be divided into medical and anatomical groups, with the anatomical contraindications being more common. The major anatomical contraindications include a dissection proximal to left subclavian artery, an insufficient proximal or distal landing zone (Fig. 30.3A,B), an inadequate diameter, severe angulation, and calcification or tortuosity of the vessels. Medical contraindications are few and include degenerative connective tissue disorders, untreatable contrast reactions, and certain allergies amongst others. In patients with known connective tissue disorders, endovascular therapy may provide a bridge to completion open reconstruction.
Computed tomography angiography (CTA) is currently the diagnostic modality of choice. It is a readily available, quick, noninvasive study with a high sensitivity and specificity for diagnosing aortic dissections. CTAs provide excellent imaging of the true and false lumens, which allow for approximation of entry tear sites and aid in planning of the repair.
After the diagnosis is made, initial management of a patient with a cTBAD consists of optimizing the medical therapy. This most crucial first step is prompt blood pressure control and anti-impulse therapy (i.e., esmolol and nitroprusside). Any form of operative therapy in the setting of uncontrolled hypertension can lead to further complications and/or suboptimal results. It is beneficial to collaborate with intensivists in the perioperative period to assist with these issues.
TEVAR should be performed in a hybrid operating room with skilled vascular, interventional, and anesthesia teams. Patients should receive a prophylactic dose of intravenous antibiotics within 1 hour of skin incision. This procedure can be performed under general, regional, or local anesthesia depending on the patient’s comorbidities and anesthesia risk. A preprocedural pulse check should also be performed and verified at the end of the procedure. The surgeon should also verify that all the necessary equipment is available and ready for the procedure. This includes ensuring that there is a working fluoroscopy machine, intravascular ultrasound (IVUS), and that all the necessary catheters, wires, grafts, and stents are readily available.
Treatment Strategies
Different strategies are applied in the treatment of dissections depending on their level of acuity and whether or not a rupture is present. These strategies need to be considered prior to the actual surgery so that the goals of treatment can be accomplished. In treating acute cTBADs with rupture (Fig. 30.4A,B




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree