8 Tetralogy of Fallot with Pulmonary Atresia
I. CASE
A. Fetal echocardiography findings
1. The fetal echo reveals situs solitus of the atria, levocardia, right aortic arch, heart rate 149 bpm.
2. Cardiac axis, position, and size are normal (cardiothoracic ratio = 0.29).
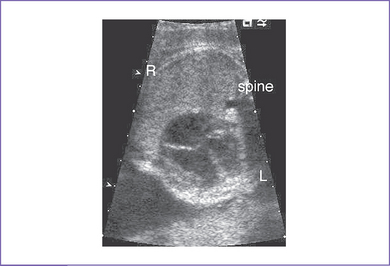
3. The four-chamber view is normal, with an axis that is approximately 90 degrees (Fig. 8-1). However, more superior scanning shows it to be abnormal, with a large outlet perimembranous subaortic ventricular septal defect (VSD) and a large overriding aorta.
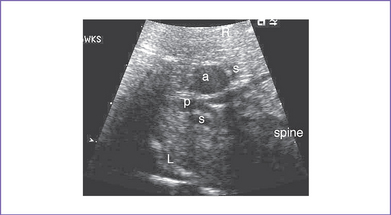
4. The outflow assessment reveals normally related great arteries with significant asymmetry (aorta–to–pulmonary artery diameter ratio = 5.5:1.5).
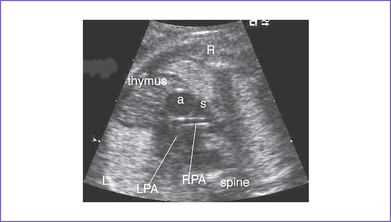
5. There is a blind outlet of the right ventricle (RV) connected to a small main pulmonary artery with hypoplastic and confluent branches (Fig. 8-2).
6. The foramen ovale is patent, with unrestricted right-to-left shunt.
7. There is reversed ductal flow (aorta to pulmonary), and multiple small aortopulmonary collaterals come from the thoracic aorta.
8. The pulmonary venous flow pattern is normal.
9. Tei index (myocardial performance index).
a. Left ventricular (LV) Tei index is normal.
b. RV Tei index could not be calculated because of the atretic pulmonary valve.
D. Fetal management and counseling
1. Diagnosis of tetralogy of Fallot (TOF) with pulmonary atresia (or pulmonary atresia and VSD) should prompt referral (Fig. 8-3).
a. Thorough anatomic examination by ultrasound.
2. Follow-up includes serial antenatal studies at 4- to 6-week intervals.
a. The branch pulmonary arteries may be hypoplastic or discontinuous. This will affect the surgical management of the baby postnatally (Fig. 8-4).
b. Absence of antegrade flow should be documented across the main pulmonary artery. In pulmonary atresia, there is no patent connection between the right ventricle and the pulmonary arteries.
c. Reversal of ductal flow suggests that pulmonary artery blood supply is from the arterial duct (pulmonary atresia with duct-dependant circulation).
d. Descending aorta to pulmonary artery collaterals should be identified. The presence of these collaterals can increase the likelihood of 22q11.2 deletion, based on postnatal experience.
e. Development of hydrops fetalis is uncommon in fetal TOF and pulmonary atresia, unless there is chromosomal abnormality not related to structural heart defect or inadequate cardiac output.
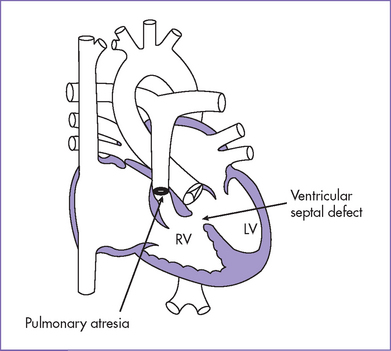
Fig. 8-3 Tetralogy of Fallot with pulmonary atresia with a small main pulmonary artery.
(Modified from Mullins CE, Mayer DC: Congenital Heart Disease: A Diagrammatic Atlas. New York, Liss, 1988.)
F. Neonatal management
a. Prostaglandin E1 (PGE1) infusion should be started to keep the ductus open to increase pulmonary blood flow and raise arterial oxygen saturation.
b. If there is no ductus arteriosus, or if it is uncertain whether the branch pulmonary arteries themselves are stenotic or if the pulmonary arteries are discontinuous with uncertain source of pulmonary blood flow, the infant requires cardiac catheterization.
c. If pulmonary blood flow is via aortopulmonary collaterals and the infant has sufficient oxygen saturations, some centers do not perform cardiac catheterization in the neonatal period, but rather closer to the time of repair with unifocalization of the collaterals, perhaps at 2 to 3 months.
d. Administration of oxygen can increase oxygen saturation by decreasing pulmonary vascular resistance and increasing blood flow.
e. At times, volume and inotropic support are indicated to improve ventricular function and increase the pulmonary blood flow through raising the systemic vascular resistance.
b. Staged repair consists of the initial systemic pulmonary shunt to encourage growth of the central pulmonary artery before primary repair.
c. When the central pulmonary arteries are nonconfluent, with multiple collaterals supplying different segments of the lungs, unifocalization of these collaterals may be necessary at the time of complete repair or with staged reconstruction.
d. Occlusion of the systemic collateral arteries can be done by coil embolization preoperatively (transcutaneous during cardiac catheterization or intraoperatively while off cardiac bypass) and would be considered if there is an antegrade source of pulmonary blood flow through the native branch pulmonary arteries.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree