7 Tetralogy of Fallot
I. CASE
A. Fetal echocardiography findings
1. The fetal echo reveals situs solitus of the atria, levocardia, right aortic arch, and heart rate 122 bpm.
2. The four-chamber view is normal, with equal sized ventricles.
3. The cardiac axis is abnormal (more toward the left, 90 degrees).
4. Position and size are abnormal (cardiothoracic ratio = 0.32).
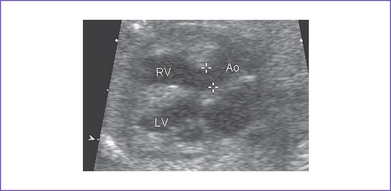
5. There is a moderate-size perimembranous subaortic ventricular septal defect (VSD), large overriding aorta, and anterior malalignment of the conal septum with subpulmonary narrowing (Fig. 7-1).
6. There is a small main pulmonary artery with confluent branches.
7. The right ventricular outflow tract (RVOT) velocity is 1.4 m/s.
8. The pulmonary artery annulus is 2 mm; the aortic artery valve annulus is 3 mm.
9. Ductal flow is normal (right to left).
10. The foramen ovale is patent, with unrestricted right-to-left shunt.
11. There is a left aortic arch and ductal arch, which are of similar size. Both arches have antegrade flow.
12. The pulmonary venous flow pattern is normal.
13. Tei index (myocardial performance index) in both ventricles is normal.
D. Fetal management and counseling
1. Management: Diagnosis of tetralogy of Fallot (TOF) should prompt referral for the following:
a. Thorough anatomic examination by ultrasound.
a. Serial antenatal studies were made at 6-week intervals.
b. If a significant gradient develops through either the VSD or the RVOT with high blood velocities, restriction of the VSD should be excluded.
c. Development of hydrops fetalis is uncommon in fetal TOF unless there is a chromosomal abnormality (not related to structural heart defect) or restriction of the VSD.
d. Congestive heart failure (CHF) can develop with:
F. Neonatal management
b. Classic TOF with pulmonary stenosis.
a. Hypoxic spells should be recognized and treated appropriately.
b. Oral propranolol 2 to 4 mg/kg per day may be used to prevent hypoxic spells and delay corrective surgery.
a. Palliation: In infants with severe RV outflow obstruction and cyanosis or uncontrollable hypoxic spells in whom corrective surgery cannot be performed, palliation in the form of a modified Blalock–Taussig shunt may be created using a Gortex tube to anastomose the subclavian artery or brachiocephalic artery and the ipsilateral pulmonary artery.
G. Follow-up
1. The baby should be monitored routinely with echocardiography for the development of RV dysfunction, significant pulmonary insufficiency, and pulmonary outflow tract obstruction.
2. Varying levels of activity limitation may be indicated.
3. Prophylaxis for subacute bacterial endocarditis (SBE) should be used as indicated.
4. Arrhythmias, particularly ventricular tachycardia, can develop later and can cause sudden death. Holter monitoring, antiarrhythmic agents, and pacemaker therapy may be indicated.
5. Pulmonary valve replacement is needed in up to 20% of patients. The timing of and criteria for this intervention are currently being developed.
6. RV–to–pulmonary artery conduit follow-up.
a. Subsequent interventional catheterization(s) with balloon dilation and stent placement in the conduit may be needed to relieve significant conduit stenosis.
b. Surgical replacement of the conduit should be anticipated.
7. Branch pulmonary artery stenosis (diagnosed by cardiac magnetic resonance imaging [MRI] and lung isotope scan) can require postoperative balloon angioplasty with stent placement or surgical repair. The aim is to normalize blood flow to both lungs, thus reducing the RV systolic pressure. These additional procedures carry a risk of morbidity and mortality.
H. Risk of recurrence
1. The fetal karyotype done on amniocytes showed a male fetus with trisomy 21 (47,XY,+21).
2. The estimated recurrence risk of TOF in a fetus with normal karyotype, including FISH for 22q11.2 deletion, is 1.5% to 2%.
3. When the baby has trisomy 21 or other underlying condition, the recurrence risk is determined by the underlying etiology.
I. Outcome of this case
1. The baby was born vaginally at term with a good Apgar score and good weight.
2. Mild cyanosis was noted at birth; pulse oximeter reading was 90% in room air. There was no need for the hyperoxia test because cyanotic heart disease was diagnosed during gestation.
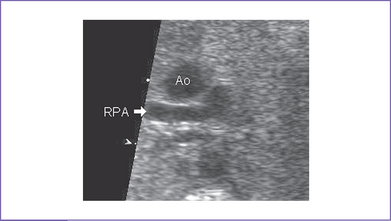
3. Postnatal echocardiography confirmed the diagnosis of TOF with large subpulmonary VSD with posterior deviation of the outlet septum causing valvar and subvalvar pulmonary stenosis. The pulmonary arteries were of good size, with a transpulmonary Doppler velocity of 4.5 m/s (Fig. 7-3).
4. The foramen ovale and ductus arteriosus were patent and of a good size.
5. In view of the baby’s anatomy and the current degree of cyanosis, prostaglandin E1 (PGE1) infusion was not started. It was decided to let the ductus arteriosus start to close and then decide based on the degree of cyanosis.
6. The baby maintained a pulse oximeter saturation of 85% to 90% and was feeding well, so he was discharged home and followed monthly at the cardiology outpatient clinic. The mother was made aware of the possibility of cyanotic spells.
7. The baby had successful surgical repair (patch closure of the VSD, widening of the RVOT, and pulmonary valvuloplasty) at 4 months of life.
II. YOUR HANDY REFERENCE
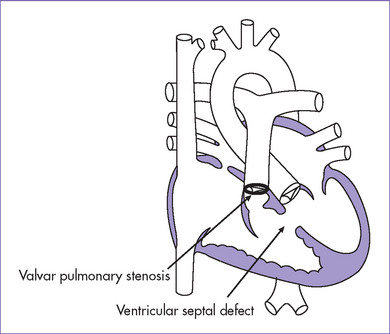
A. Tetralogy of Fallot (Figs. 7-4 and 7-5)
a. TOF accounts for 10% of all congenital heart disease. It is the most common form of cyanotic heart disease beyond infancy.
b. TOF is one of the more commonly encountered forms of heart disease in the fetus, and in one series it is the third most commonly identified form of structural heart disease. This could be due to the very common occurrence of aneuploidy and extracardiac structural pathology rather than recognition of the pathology at routine obstetrics assessment.
c. Classic TOF can be missed if echocardiographic examination of the fetal heart is confined to the four-chamber view, because an abnormal four-chamber view is rarely observed in this condition. One might see an abnormal four-chamber view in tetralogy with absent pulmonary valve, with mitral valve obstruction, or with restrictive VSD, all of which are less common than the classic form of TOF.
d. Allan and Sharland (1992) studied a total of 125 cases of TOF diagnosed prenatally. They found:
a. Two studies (Berning and colleagues [1996] and Hornberger and colleagues [1999]) have shown that perinatal mortality may be as high as 35% to 75%.
b. The perinatal outcome of fetal TOF is worse than that observed for postnatally identified TOF. The possible explanation is the relatively high incidence of aneuploidy and extracardiac anomalies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree