Testing for Viable Myocardium
Arun Dahiya
I. INTRODUCTION.
Patients with left ventricular (LV) dysfunction secondary to coronary artery disease (CAD) have significant morbidity and mortality. Given the prognostic implications of poor ventricular function, it is imperative to identify any reversible myocardial dysfunction that may improve with revascularization.
II. DEFINITIONS
A. Viable myocardium
is defined as myocardium that demonstrates abnormal function at rest and improves with revascularization.
1.From a pathophysiologic standpoint, chronically reduced perfusion leads to cellular changes that ultimately cause irreversible myocyte dysfunction.
2.Biopsies of myocardium reveal a spectrum of fibrosis and sarcomere loss that correlates with the likelihood of recovery of function. Several studies have found that once fibrosis is found in more than 35% of the myocardium, the likelihood of recovery of function is low.
3. Stunning refers to transient myocardial dysfunction, which is often caused by abrupt cessation of flow typical of an acute coronary occlusion.
4.Myocardial scar from cellular necrosis is irreversible and does not improve with revascularization.
B. Hibernation
occurs when viable myocardium has altered its metabolism and thus reduced its contractile function as a mechanism to cope with chronically inadequate blood supply (chronic stable angina) or repetitive ischemic injury.
III. CLINICAL PRESENTATION.
Ischemia, stunning, hibernation, scarring, and normal myocardium may coexist in the same patient. Unfortunately, clinical symptoms are unreliable in determining if a patient has viable myocardium, as often patients experience no symptoms in the face of considerable LV dysfunction and ischemia.
IV. TREATMENT OPTIONS.
It has been demonstrated that revascularization of viable myocardium improves quality of life and survival. As medical and surgical technology improves in the field of cardiovascular medicine, it is important to accurately identify patients who will benefit from revascularization.
A. Thrombolytic therapy or emergency percutaneous revascularization
is used in the setting of an acute thrombotic occlusion to restore normal blood flow and hopefully to minimize cellular damage.
B. Revascularization procedures,
such as coronary artery bypass grafting and percuta neous transluminal coronary angioplasty, may improve regional and global LV systolic function caused by significant CAD. The presence and extent of viable myocardium have been demonstrated as a marker for patients who will do significantly better with revascularization than with conventional medical care.
C.
It is notable that patients with nonviable myocardium have similar outcomes with medical therapy as with revascularization.
D.
Because not all patients benefit from revascularization procedures, the identification and referral of patients who will derive benefit are important to reduce costs and morbidity with the associated procedures. The goal, therefore, is to reliably identify patients who will benefit from revascularization and subsequently refer these patients for appropriate intervention.
V. TECHNIQUES TO ASSESS VIABILITY
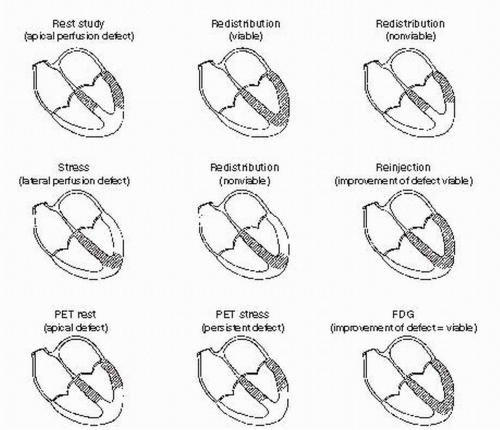
(Fig. 50.1). Assessment of myocardial viability is indicated in patients with CAD and resting LV dysfunction who are eligible for revascular-ization. Coronary angiograms provide information about anatomy and feasibility of revas-cularization but do not predict recovery of function. Resting echocardiography provides information regarding overall LV function and segmental wall motion abnormalities but does not address recovery of function with revascularization techniques. Single photon nuclear imaging techniques (single photon emission computed tomography, SPECT), positron emission tomography (PET) with a metabolic agent, dobutamine echocardiog-raphy, contrast echocardiography, and, more recently, delayed-enhancement magnetic resonance imaging (MRI) have been identified as techniques that can distinguish viable myocardium from nonviable myocardium. Each technique exploits a separate property of dysfunctional myocardium to determine the potential for recovery of function after revas-cularization. The test that is used often depends on the strengths and preference of each medical center, although an approach based on the individual patient would be preferable.
A. Single photon emission computed tomography.
SPECT is the most common technique used in the United States to identify viable myocardium. This technique has been successful because thallium 201 and technetium 99m radiopharmaceuticals act as perfusion agents that are only taken up by viable tissue. The long half-lives of these agents allow for regional distribution, which makes them feasible to use in medical centers without a generator or cyclotron. In addition, stress SPECT protocols (exercise or pharmacologic) are frequently used to assess for ischemia, which makes this technique cost-effective in a busy clinical center. Routine studies also include gated imaging analyses that provide further information regarding LV function and wall motion assessment, which are important in the evaluation of viability.
1. Thallium 201
is a potassium analogue that utilizes the Na+/K+-ATPase active cellular transport system for concentration in cells and relies on intact cells. This characteristic of thallium makes it useful for the identification of viable cells as opposed to necrotic cells. Uptake of thallium is also dependent on regional myocardial perfusion.
a.Thallium 201 has a relatively long half-life (73 hours), which means that a small dose (2 to 4 mCi) must be used. It emits x-rays from 68 to 80 keV (94% abundant) and γ-rays at 135 and 167 keV (10% abundant). There is a linear relationship between blood flow and uptake of thallium at rest, which is maintained with exercise making it as a reliable indicator of perfusion.
b. Redistribution of thallium between the intracellular and intravascular spaces begins to occur after the first pass of thallium. It is recognized that with time, initial defects on thallium imaging improve. This is thought to be related to the accumulation of tracer in hypoperfused areas over time as well as rapid washout in normally perfused areas. On the basis of these principles, several protocols have been used to identify viable myocardium.
(1) Rest/redistribution thallium imaging involves imaging 30 to 60 minutes after an initial injection followed by reimaging 4 hours later. Defects on the initial images that improve in 4 hours are considered to represent areas of viable myocardium. This protocol does not address ischemia and has been proven to be less sensitive for detecting viable myocardium than other protocols using thallium or PET with 18F-fluorodeoxyglucose (FDG).
(2) Stress/redistribution imaging uses pharmacologic or exercise-induced stress with subsequent thallium injection and imaging, immediately followed by reimaging 4 hours later. Myocardium that is not perfused with stress or rest is considered to be scar. Myocardium that has a defect with stress but that improves on rest images is considered to be ischemic and viable. This protocol can identify ischemic viable myocardium, but it also shows lower sensitivity than other protocols, as many of the defects that do not improve at 4 hours may contain viable tissue. Imaging 24 hours after stress in a search for “late redistribution” improves sensitivity in the detection of viability but has low specificity and may be inconvenient. However, blood levels of thallium 201 may still be too low to be redistributed and picked up by viable myocardium. This led to the development of reinjection protocols mentioned later in this chapter.
(3) Stress/redistribution/reinjection protocols involve the reinjection of 1 mCi of thallium with subsequent reimaging of the patient. This protocol is designed to increase the sensitivity by increasing the blood levels of thallium. It was shown that 50% to 70% of “scarred myocardium” after 4-hour redistribution imaging was actually viable, as demonstrated by this technique. Typically, reinjection of thallium and repeated imaging is performed immediately after the redistribution images or several hours after the initial stress images, followed by redistribution imaging 18 to 24 hours later. Sensitivity does not differ significantly between these two techniques. Scar is considered to be myocardium that has a defect on the stress images and does not improve upon reinjection and reimaging. Viable myocardium is indicated by uptake of tracer on reinjection in segments where no uptake occurred with stress.
2. Technetium 99m-labeled radiopharmaceuticals
rely on mitochondrial func tion, sarcolemmal integrity, and intact energy production pathways for cellular accumulation.
a. Technetium characteristics. Technetium 99m compounds have a shorter half-life (6 hours) than thallium, which allows for the administration of
larger doses (10 to 30 mCi), depending on the compound. Technetium 99m emits γ-rays at 140 keV. The commonly used agents include Tc 99m sestamibi, Tc 99m tetrofosmin, Tc 99m teboroxime, Tc 99m furifosmin, and Tc 99m NOET (bis(N-ethoxy, N-ethyl dithiocarbamato)nitrido). b. Redistribution of technetium compounds is significantly less than that for thallium, making it relatively unhelpful as an aid in detecting viability.
larger doses (10 to 30 mCi), depending on the compound. Technetium 99m emits γ-rays at 140 keV. The commonly used agents include Tc 99m sestamibi, Tc 99m tetrofosmin, Tc 99m teboroxime, Tc 99m furifosmin, and Tc 99m NOET (bis(N-ethoxy, N-ethyl dithiocarbamato)nitrido). b. Redistribution of technetium compounds is significantly less than that for thallium, making it relatively unhelpful as an aid in detecting viability.