Rewarming
The patient must be rewarmed if hypothermia is used for organ protection (
1). Mild hypothermia (33°C-35°C) is used for shorter cases with warm intermittent or continuous cardioplegia. Moderate hypothermia (25°C-30°C) is often used to slow myocardial rewarming after cold cardioplegia. Deep hypothermia (18°C-22°C) with circulatory arrest is occasionally needed for repairs of certain congenital defects or for aortic arch reconstruction. Rewarming with the heat exchanger in the bypass circuit is initiated to restore normothermia by the completion of the surgical procedure. Temperature measurement of the arterial and venous blood is necessary to keep the arteriovenous gradient less than 6°C to prevent bubble formation, as the blood warms and gaseous solubility decreases (
2). The temperature required for complete rewarming is usually a nasopharyngeal or esophageal temperature of 37°C or a bladder or rectal temperature of 35°C to 36°C. Palpation of the patient’s head and shoulders can be helpful in assessing the degree of rewarming. However, the high blood flow-totissue mass ratio of the head and neck region may result in underestimation of the completeness of rewarming in other vascular beds. Therefore, sweating of the forehead during rewarming in CPB does not necessarily mean that the patient is “lightly anesthetized” but suggests a normal thermoregulatory response to rewarming (
3).
Inadequate rewarming after discontinuation of CPB can result in rebound hypothermia. This problem is often compounded by further convective heat loss from the patient into the cool operating room environment. Pharmacologic-induced vasodilation during CPB may facilitate rewarming and decrease the postbypass fall in temperature (
4). However, the use of this technique may require more intravascular volume expansion to maintain adequate perfusion pressure, which worsens hemodilution and tissue edema. Inadequate rewarming while on CPB can result in a considerable (2°C-3°C) drop in patient temperature from the end of CPB until arrival in the intensive care unit. This fall in temperature can result in shivering, which increases oxygen consumption, carbon dioxide production, and peripheral vascular resistance. Subclinical shivering in a patient who is partially paralyzed during CPB results in hypercarbia and the need for muscle relaxation. Shivering should be anticipated when systemic temperature is less than 35°C. It should be aggressively treated with active rewarming measures.
Metabolic Abnormalities
Nonpulsatile flow results in vasoconstriction during CPB and a low perfusion state especially during prolonged cases that can cause metabolic problems. Acidemia, from respiratory or metabolic causes, should be corrected because of its depressant effects on myocardial function, its interference with the action of inotropic drugs, and its ability to increase pulmonary vascular tone (
5,
6,
7). Acidemia and administration of cardioplegic solutions frequently result in hyperkalemia, which has pronounced deleterious effects on cardiac conduction, including atrioventricular conduction block (
8). Atrial depolarization is especially sensitive to hyperkalemia and failure to capture either the atria or ventricle with pacing can result. Hyperkalemia can be treated with insulin, calcium, and bicarbonate. It is usually unnecessary to treat mild hyperkalemia (<6.0 mEq/L) in the presence of normal renal function, because serum potassium usually falls after CPB due to rewarming and increased glucose utilization, renal losses from a diuresis that develops from the hemodilution, and increased
catecholamine and β
2 receptor stimulation from the stress of CPB, all of which move potassium intracellularly. If hypokalemia develops after bypass, it should be treated promptly, because ventricular and atrial arrhythmias are likely to develop.
Hypocalcemia results from hemodilution and transfusion of albumin or citrate-containing blood products. Hypocalcemia during CPB should not be treated, because normocalcemia usually occurs by the end of rewarming due to the normal response of increased parathyroid hormone (
9). If ionized hypocalcemia is present (<0.8 mg/dL) after rewarming is complete, then it should be corrected with calcium chloride (5 mg/kg) to improve myocardial contractility and peripheral vascular resistance. Otherwise, calcium should not be administered, because it can worsen the reperfusion injury that occurs after cardioplegic arrest. Hypomagnesemia also results from hemodilution, but magnesium has no counterregulatory hormone to increase magnesium levels. Magnesium should be administered during CPB to provide vasodilation of coronary arteries, to attenuate post-CPB hypertension, and to prevent arrhythmias (
10). Hyperglycemia is common during the hypothermic period of CPB and usually returns to normal shortly after termination of CPB in the nondiabetic patient. However, persistent hyperglycemia after CPB in any patient should be treated with insulin. In diabetic patients, insulin infusions are often needed during CPB and postoperatively for hyperglycemic control. Even
mild hyperglycemia (>150 mg/dL) has significant adverse effects in terms of increasing the risk of postoperative infection, wound healing, and increased mortality (
11). More severe hyperglycemia increases serum osmolarity and can cause osmotic diuresis and central nervous system dysfunction and may increase the susceptibility of the brain to hypoxic damage.
Pump priming with crystalloid solutions results in hemodilution. Hemodilution reduces blood viscosity and improves microcirculatory flow; however, hemodilution decreases serum protein levels, which decreases plasma colloid osmotic pressure and increases the movement of fluid from the intravascular to the extravascular compartments to cause tissue edema. Hemodilution reduces hemoglobin and hematocrit concentrations. The optimal hemoglobin concentration during CPB is usually accepted as being ≥ 7 g/dL, although there is no proven minimum safe level. However, Hgb <8 g/dL has been associated with worsened outcomes, yet treatment with blood transfusion makes outcomes even worse (
12). Healthy adult patients rarely need homologous blood transfusion with appropriate blood conservation measures if they are not anemic preoperatively. Blood transfusion increases the risk of renal failure and infection; so the benefits of blood transfusion in terms of increased oxygen transport should be weighed against its risks.
Heparin-induced anticoagulation should be carefully monitored during rewarming because of the increased metabolism of heparin at higher body temperatures and the dangerous consequences of inadequate anticoagulation. In addition to reducing electrolyte levels and hematocrit, hemodilution reduces platelet levels by about 40% and dilutes coagulation factor levels (
13). Protamine should be available to reverse heparin following termination of bypass but some recommend protamine not be drawn into a syringe until needed to avoid the possibility of administration while on cardiopulmonary bypass (CPB). Plasma, cryoprecipitate, or prothrombin complex concentrates should be available for treating documented factor deficiencies or coagulopathy. DDAVP may improve platelet function in patients with aortic stenosis or a ventricular assist device who develop an acquired von Willebrand factor deficiency (
14). Platelet transfusions are occasionally required, especially in patients who have significant thrombocytopenia (<50 K), chronic renal failure, or who are receiving antiplatelet drugs. Postoperative bleeding is usually due to inadequate surgical hemostasis, inadequate heparin reversal, or mild coagulopathy.
Anesthesia, Oxygenation, and Ventilation
The requirements for anesthetics and muscle relaxants are reduced during CPB in approximate proportion to the level of hypothermia because of anesthetic effects of hypothermia and decreased metabolism of most drugs (
15). Changes in intravenous anesthetic drug distribution and elimination also contribute to reduced anesthetic requirements. However, rewarming reverses much of this effect, and hence adequate anesthetic depth and muscle relaxation to prevent patient awareness and shivering becomes a concern. As a result, benzodiazepines, narcotics, and muscle relaxants may be needed during rewarming in anticipation of volatile agents being discontinued before termination of CPB to avoid their circulatory and myocardial depressant effects (
16,
17). Administration of volatile anesthetic agents during the weaning process can contribute to failure to wean from CPB because of cardiodepressant effects. However, volatile anesthetic agents also induce vasodilation and facilitate rewarming and may be continued during rewarming, but most often should be discontinued approximately 10 minutes before termination of bypass.
Oxygenation and ventilation must begin before discontinuation of bypass. The lungs should be manually reinflated and visually inspected to document bilateral reinflation and elimination of atelectasis. Unilateral reinflation suggests malposition of the endotracheal tube or possibly mucus plug or blood clot in a mainstem bronchus, which may require therapeutic bronchoscopy. Suctioning should be done carefully in the anticoagulated patient to avoid mucosal trauma and bleeding. Occasionally, a pneumothorax or blood accumulation in the pleural space may impede reinflation, both of which are easily corrected by the surgeon. Mechanical ventilation with 100% oxygen and a sufficient minute ventilatory volume to produce an arterial P PCO
2 of 30 to 35 mmHg should be initiated before beginning the weaning process. Minute ventilation should be higher than pre-CPB because of the increased carbon dioxide production during rewarming, which extends into the post-CPB period. Mild hyperventilation prevents hypercapnia and respiratory acidosis, which could result in elevated pulmonary artery pressures (PAPs) (
18,
19). Patients with chronic lung disease may sometimes require positive end-expiratory pressure (PEEP), pressure controlled ventilation, or bronchodilators.
Most CPB consoles include an in-line monitor of mixed venous blood hemoglobin oxygen saturation. Changes in this parameter may reflect the adequacy of systemic perfusion and oxygen delivery or increases in oxygen consumption (e.g., shivering) while on CPB. Before terminating CPB, the venous saturation and pump flow should be checked. A low saturation may indicate the need for additional muscle relaxant, red blood cell transfusion, or vasodilator therapy before weaning from bypass. Substantial reduction in extracorporeal circuit venous oxygen saturation during the weaning process strongly suggests inadequate cardiac output for successful separation from CPB.
Hemodynamic Monitors
All routine monitors need to be returned to their full operating mode before terminating CPB. Pulse oximeter probes placed on the extremities often do not function while the patient is cold and pulseless on CPB. Assessment of their function must wait until partial bypass is instituted. Pressure transducers
should be re-zeroed. Frequently, the radial arterial pressure does not reflect the central aortic pressure after CPB (
20,
21,
22). If there appears to be a discrepancy between the transduced radial arterial pressure and the aortic pressure estimated by palpation or the blood pressure cuff, the aortic pressure can be transduced from the antegrade cardioplegia line or stopcock on the aortic cannula once bypass is discontinued. Documenting an adequate central aortic pressure compared to an inadequate radial pressure can prevent the administration of unnecessary vasoactive drugs. Alternatively, a femoral arterial catheter, which will more closely reflect the central aortic pressure, can be placed. Central and peripheral arterial pressures usually equilibrate shortly after CPB is discontinued.
Pulmonary artery catheters frequently migrate distally during manipulation of the heart during cardiac surgery (
23). If the PA waveform reflects pulmonary artery occlusion pressure, the catheter should be pulled back while on partial bypass until the phasic PAP waveform reappears. A left atrial (LA) catheter can be a useful monitor of left ventricular preload if a pulmonary artery catheter is not present and transesophageal echocardiography (TEE) is not being used. An LA catheter can also be used as a route for administration of inotropic drugs, especially those with significant α-adrenergic agonist properties in the context of excessive pulmonary vasoconstriction (see subsequent text). The central venous pressure (CVP) should be measured, especially in those patients with pulmonary hypertension or right ventricular failure. Appropriate right/left ventricular function balance is an important variable in successful weaning.
Urine output is routinely monitored during CPB but low urine output during CPB is not independently predictive of postoperative renal dysfunction (
24). Nevertheless, for patients with preoperative renal dysfunction or low urine output during CPB higher perfusion pressures during CPB should be considered (e.g., mean arterial pressure [MAP] >60 mmHg). Some cardiac surgical centers may have protocols for renal protection, which include volume loading when possible to establish good urine output pre-CPB, bicarbonate infusions, or additional mannitol; no renal protection protocol has been proven to protect renal function during CPB. After resumption of pulsatile blood flow, many patients exhibit a marked diuresis due to the hemodilution, particularly if mannitol has been administered.
TEE provides information that can be very useful during termination of CPB. While on partial CPB, TEE provides an excellent assessment of residual air in the heart after open procedures and allows de-airing maneuvers which can prevent a coronary air embolus post-CPB. The left ventricular short-axis view provides a useful indicator of left ventricular size and filling. Regional wall motion abnormalities may provide an important indicator of myocardial ischemia and inadequate flow through a specific coronary artery bypass graft (CABG). However, regional wall motion abnormalities may also be due to residual effects of cardioplegia, inhomogeneous myocardial temperature, or preexisting abnormalities. One of the most useful applications of TEE before and after the termination of CPB is in evaluating valvular function after valve replacement or repair (
25). Epicardial echocardiography and TEE have been proven useful in evaluating the repair of complex congenital heart defects (
26,
27). Also, in contrast to routine intracardiac pressure measurements, which do not accurately reflect cardiac chamber volumes, TEE provides the capability to accurately assess biventricular filling volumes (
28,
29). Direct observation of the right ventricle (RV) on the anterior surface of the heart is a useful monitor for guiding volume infusions from the pump and can prevent the right heart from becoming overdistended.
Heart Rate and Rhythm
After the aortic cross-clamp is released and coronary reperfusion commences, cardiac electrical activity returns. This may be in the form of ventricular fibrillation, which is likely a reperfusion arrhythmia due to calcium overload of ischemic myocardium. Lidocaine is often given before the cross-clamp is released or in the cardioplegia and is effective at preventing ventricular fibrillation. Ventricular fibrillation during CPB may result in ventricular distension and irreversible myocardial damage. The heart should be electrically defibrillated as soon as possible. Electrolytes should be treated if abnormal. Recurrent ventricular fibrillation should be treated with amiodarone and repeat defibrillation. β-Adrenergic blockers are also remarkably effective for facilitating defibrillation in resistant cases.
More commonly after hyperkalemic cardioplegic arrest, the return of cardiac electrical activity is in the form of a junctional bradycardia or sinus bradycardia with atrioventricular conduction block. Sinus bradycardia is easily treated with atrial pacing when normal A-V conduction is present. Sequential atrioventricular pacing is indicated for atrioventricular conduction block or significant first-degree heart block. This preserves the atrial contribution to ventricular filling, which is a significant advantage in the presence of a noncompliant hypertrophied ventricle (hypertension, aortic stenosis) or enlarged ventricle (aortic or mitral regurgitation). Ventricular pacing should be used only when atrial or atrioventricular pacing is not feasible (e.g., atrial fibrillation or flutter with a very slow ventricular response) or in a backup demand mode when the patient is in sinus rhythm. In patients with low ejection fraction and preoperative conduction system abnormalities, temporary biventricular pacing may improve postoperative hemodynamics and prevent left ventricular dyssynchrony (
30).
In the absence of TEE, all available electrocardiogram (ECG) leads should be examined and compared with a preoperative tracing before terminating CPB to detect evidence of acute myocardial ischemia. Temporary pacing can be momentarily discontinued to make this evaluation. Transient ST-segment elevation is common during emergence from CPB
but usually resolves shortly thereafter (
31). Persistent ST-segment abnormalities suggest myocardial ischemia, which may require surgical treatment (i.e., revision of a graft or placement of an additional graft). Intracoronary air embolism usually involves the right coronary artery, which should resolve after a short period of increased perfusion pressure. Coronary artery or internal mammary artery spasm also produces a similar clinical picture and often responds favorably to treatment with intravenous nitroglycerin or to elevation of the perfusion pressure.
Tachycardia before termination of CPB is more difficult to manage. Heart rate often slows with ventricular filling and increased arterial pressure; therefore termination of CPB may slow sinus tachycardia. It is important to consider and treat other common causes of tachycardia, including hypoxemia, hypercapnia, anemia, inadequate anesthesia, and inotropic drugs. Once these causes are eliminated and myocardial function is determined to be adequate, the abnormal rate can be reduced with appropriate doses of β-adrenergic receptor or calcium channel blocking drugs. The b receptor antagonist esmolol is especially useful because of its short duration of action if adverse effects develop. Refractory supraventricular tachycardia, atrial fibrillation, or flutter is best treated with electrical cardioversion.
Ventricular Function and Prophylactic Inotropic Support
The ischemia imposed by aortic cross-clamping can result in considerable myocardial stunning, which is prolonged reversible acute postischemic ventricular dysfunction of viable myocardium that follows reperfusion (
32). In the case of chronic myocardial ischemia, successful myocardial revascularization may improve ventricular function by restoring perfusion. However, improvements in ventricular function can be delayed for hours to days because of myocardial stunning (
33). Improved ventricular function is usually obtained after aortic valve replacement for aortic stenosis where the compensatory response to chronic pressure overload is concentric hypertrophy of the ventricular wall that tends to normalize wall stress (
34). In contrast, myocardial dysfunction is frequently present after valve replacement in the patient with mitral regurgitation where removing the low-pressure ejection of blood into the left atrium increases left ventricular afterload (
34,
35). The degree of postoperative dysfunction is less predictable in patients with mitral stenosis or aortic regurgitation, but myocardial depression may exist because of the cardiomyopathy of rheumatic heart disease or eccentric ventricular hypertrophy, respectively. Previous myocardial infarction is an obvious cause for irreversible myocardial dysfunction postoperatively. Procedures requiring a left ventriculotomy may have a profound impact on postoperative ventricular function because of transection of important coronary artery branches, resection of viable myocardium, or reduced ventricular compliance produced by left ventricular resection.
Prediction of the need for pharmacologic support after CPB is usually assessed by reviewing preoperative and pre-CPB hemodynamic data and the intraoperative course. Information such as the preoperative ejection fraction, left ventricular filling pressures before and after contrast injection during the catheterization, history of heart failure, pre-CPB cardiac index, the effectiveness of intraoperative myocardial protection, the length of time on CPB, and the adequacy of surgical repair all can affect the decision to start inotropic drugs prophylactically. The goal of prophylactic administration is to allow smooth separation from CPB without causing cardiac distension, hypotension, and reinstitution of bypass.
In a Canadian study of 12,471 patients undergoing CABG surgery, preoperative left ventricular function was important in predicting outcome. In patients with a left ventricular ejection fraction greater than 40%, the predictors of postoperative mortality were emergency surgery, female gender, reoperation, type of myocardial protection (blood-based cardioplegia appeared to offer greater protection than crystalloid cardioplegia), and age. The presence of left main coronary artery disease was a predictor of death in patients with an ejection fraction greater than 40%. The type of myocardial protection used remained correlated with mortality in patients with ejection fraction between 20% and 40% (
36). In patients with an ejection fraction less than 20%, emergency surgery was the only predictor of death (
Table 25.2).
Relatively few studies have been conducted to help predict which patients will require pharmacologic support during and immediately after CPB. In a retrospective analysis of patients who underwent CABG surgery, predictors of the need for inotropic support were essentially the same as those identified for major morbidity and mortality (
37). Preexisting low ejection fraction, a dilated left ventricle, an elevated left ventricular end-diastolic pressure (LVEDP), longer duration of aortic cross-clamp (or total bypass) time, age, and
female gender were predictors of the need for postoperative inotropic support. Interestingly, in patients with normal preoperative ejection fractions (EF > 55%), the presence of wall motion abnormalities and an LVEDP greater than 10 mmHg indicated a higher risk for needing inotropic support similar to patients with lower ejection fractions (
37). In another study, left ventricular dysfunction, defined as an ejection fraction less than 40%, LVEDP more than 15 mmHg, and abnormal systolic and diastolic left ventricular volumes, correlated with the need for postoperative hemodynamic support, either pharmacologic or with an intra-aortic balloon pump (IABP) (
38) (
Fig. 25.1).
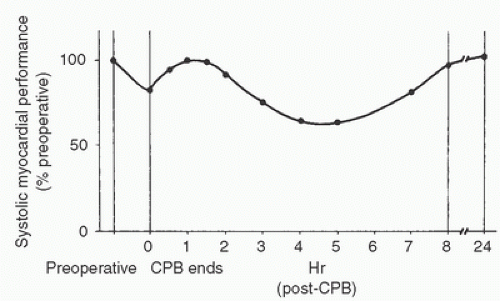
Several factors, including microemboli, reperfusion injury, chest closure, incomplete rewarming, and hemodilution, may explain the observed postoperative decline in cardiac function. One study evaluated patterns of left ventricular functional recovery after cardiac surgery. Patients with normal preoperative ventricular function recovered completely within 24 hours while reaching a peak depression in ventricular function at 4 to 6 hours after surgery (
39). In a second group of patients with reduced preoperative left ventricular function, postoperative recovery required 24 hours or more. A review summarized data from multiple studies and found that a decline in ventricular function occurs immediately after CPB as compared to preoperative ventricular function, with consistent early post-CPB improvement reaching the preoperative level 1 to 2 hours after CPB (
40). A decline in function follows that, which reaches a nadir 4 to 6 hours after CPB with subsequent improvement over the next 12 to 18 hours (
Fig. 25.1). Therefore, the intuitive predictors of needing inotropic therapy after CPB are supported by clinical data, and preoperative left ventricular dysfunction strongly predicts a requirement for postoperative inotropic support.