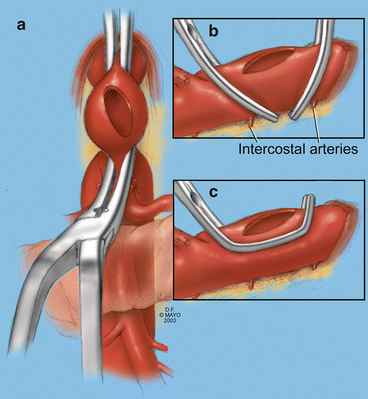
Fig. 11.1
Schematic drawing of additional upward and lateral retraction of the abdominal wall obtained incising the sic musculoaponeurotic attachments along the site of the xiphoid process (By permission of Mayo Foundation for Medical Education and Research. All rights reserved)
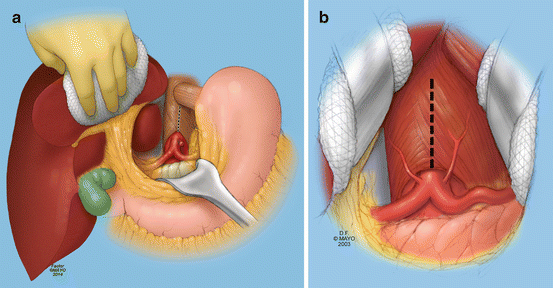
Fig. 11.2
Transperitoneal exposure of the supraceliac aorta and the celiac artery. First, the left triangular ligament of the liver is divided so that the liver can be retracted toward the patient’s right side. With a nasogastric tube in place, the stomach and esophagus are gently retracted to the left side (a). The patient can be placed in a slight reverse Trendelenburg position and the pancreas and the viscera retracted caudally to achieve exposure of the celiac. The crural fibers are divided longitudinally over the top of the aorta (b). As much as 5–8 cm of the supraceliac or lower descending thoracic aorta can be exposed through this approach (By permission of Mayo Foundation for Medical Education and Research. All rights reserved)
The celiac artery is isolated first when a two-vessel antegrade reconstruction is planned. The patient is placed in a gentle head up position (reverse Trendelenburg), and the viscera and pancreas are gently retracted caudally by retractor blades with pads beneath them. The artery can be isolated by first exposing the common hepatic artery above the pancreas, then tracing it to the celiac bifurcation. Occasionally, there are a couple of periarterial veins which cause nuisance bleeding when disrupted. The splenic artery then is isolated, which allows for circumferential dissection of the celiac to its origin. Periarterial ganglionic and fibrous tissue requires excision. One or more small branches may be present and are ligated and divided. The left gastric artery often is ligated and divided too, which helps facilitate a retropancreatic tunnel for a superior mesenteric artery (SMA) graft limb. Complete isolation of the celiac is needed when that trunk is the target for bypass, and it also allows placement of a hypogastric clamp across the lower supraceliac aorta from below (caudal) the celiac origin, if needed (see below under Technique). The celiac is not isolated if the target for bypass is the common hepatic artery.
The superior mesenteric artery may be exposed above or below the pancreas, depending on patient anatomy and the extent of disease. If the artery is isolated above the pancreas, care must be taken not to disrupt the pancreatic capsule. This approach is reserved for rare patients whose disease is isolated to the origin of the artery. In most patients, it is safer to isolate the SMA near the base of the transverse mesocolon beyond the ligament of Treitz (Fig. 11.3). The transverse colon is retracted cephalad. The small bowel is retracted to the right side of the abdomen. The SMA can usually be palpated within the proximal mesentery. There are often lymphatic and fibro-fatty tissues along the proximal SMA which require meticulous ligation and division. Within several centimeters of the SMA origin, there are a number of jejunal branches which can be isolated with Silastic loops. Care must be taken not to injure a replaced right hepatic artery during this dissection should one be present. A patient with extensive atherosclerosis in the proximal SMA needs dissection to be carried further distally in the mesentery until a softer artery is palpated. Rare patients, who have had abdominal radiation years earlier, can have calcification of almost every branch artery. A retropancreatic tunnel is created between the supraceliac aorta and the infracolic SMA for patients having an antegrade two-vessel bypass. The tunnel is made by careful blunt dissection on top of the left renal vein and just along the left anterolateral aorta. The tunnel must be free of fibrous bands and of adequate size to accommodate the graft limb. Plasma tubing is placed through the tunnel.
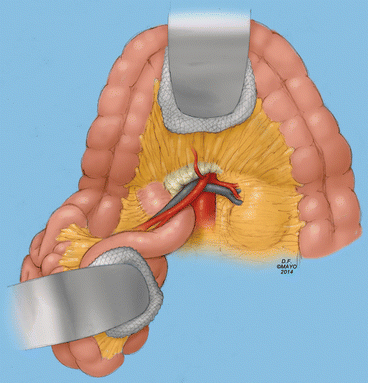

Fig. 11.3
The superior mesenteric artery is exposed at the base of the transverse mesocolon in most patients. The surgeon can palpate the artery running along the base of the small bowel mesentery. Dissection should be carried directly to the artery, which requires ligation and division of some overlying fibro-fatty tissues, lymphatics, and small veins. If there is extensive disease in the artery, the jejunal branches need to be isolated. There are some posterior branches which cause troublesome bleeding if injured (By permission of Mayo Foundation for Medical Education and Research. All rights reserved)
A midline incision is used to isolate the infrarenal aorta or iliac arteries when retrograde bypass is planned. Retrograde bypass from the iliac artery or infrarenal aorta may be preferable in patients with diseased supraceliac aortas, those with compromised cardiac or pulmonary function, and in older patients. The retroperitoneal tissues are opened vertically to isolate the aorta and iliac arteries, similar to a transperitoneal infracolic exposure for open aortic aneurysm repair.
Transaortic visceral artery endarterectomy is an infrequent but a useful technique for good-risk patients with orificial disease, those who have concomitant symptomatic renal artery atherosclerotic stenoses, and in select patients with acute mesenteric ischemia who have peritoneal contamination. Midline, extended/bilateral subcostal, or thoraco-retroperitoneal incisions can be used to perform a medial visceral rotation for exposure of the paravisceral aorta and visceral arteries (Fig. 11.4). Choice of incision is based on body habitus, costal flare, and the location of the mesenteric artery origins relative to the inferior extent of the xiphoid process on sagittal CT imaging. The left kidney is kept down. Care is taken to avoid injury to the spleen and pancreas. The aorta is isolated from the supraceliac to the juxtarenal segment in most patients, as the aortotomy must be made from above the celiac artery origin to below (caudal) the SMA origin. In patients with a short distance between the SMA and renal origins, the upper infrarenal aorta requires isolation. The left crus is divided as part of the exposure. Phrenic or other side branches are ligated and divided. An occasional patient has small aortic branches near the celiac origin which cause pesky bleeding if injured. The celiac and SMA are circumferentially dissected free for 3 or 4 cm, a key step to allow for eversion endarterectomy.
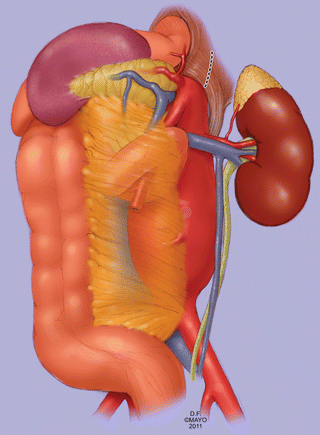

Fig. 11.4
A left medial visceral rotation is another approach to the celiac and superior mesenteric arteries, similar to the exposure used to treat patients with aneurysms which extend to the suprarenal level. This approach is useful for visceral artery endarterectomy and bypass in select patients. The left kidney is kept down. The crura of the diaphragm require division to gain access to the supraceliac aorta (By permission of Mayo Foundation for Medical Education and Research. All rights reserved)
Technique
For antegrade reconstructions based on the supraceliac aorta, the patient is given intravenous heparin (50–100 units/kg) and 12.5 g of mannitol before aortic cross-clamping (Figs. 11.5 and 11.6). The aorta either can be fully or partially cross-clamped (Fig. 11.5). Complete aortic clamping may be needed in deep patients or those with plaque in the aorta. This is best facilitated with a straight or mildly angled aortic clamp for the proximal supraceliac aorta and a hypogastric clamp for the lower supraceliac aorta. In some patients, the latter clamp can be placed from behind the celiac artery origin. This is a useful maneuver for patients with a short length of supraceliac aorta below the hiatus. This two-clamp technique allows a good view of the aortic lumen after aortotomy is made and occludes backbleeding from the lumbar arteries. Although partial aortic occlusion is preferred when possible, adequate visualization of the aortic lumen is necessary to perform the proximal anastomosis. Since this anastomosis can be done within 15 min or less in most patients, the risk of renal and lower extremity ischemia is lower than the risk of an imperfect anastomosis that requires revision. Moreover, the cardiac risk from increased afterload with complete aortic clamping should also be low with good anesthesia management. The latter requires close communication between surgeon and anesthesiologist. For these reasons, I do not hesitate to fully occlude the aorta. The author prefers an all-purpose aortic clamp to partially occlude the aorta. This clamp has deeper blades than other such clamps. Once the aorta is clamped and provided the hemodynamics remain stable, a slightly oblique aortotomy is made. A stay stitch can be placed in the aortic wall if it affords better exposure of the lumen and does not interfere with sewing. For patients with aortas of 16 mm or greater diameter, removing a sliver of aortic wall aids the view of the lumen and obviates the need for a stay stitch. If there is debris or loose plaque in the aorta, this needs to be removed to avoid distal embolization into the kidneys and/or extremities. Bypass in most patients is done with a 12 × 7 mm, 14 × 7 mm, or a 16 × 8 mm knitted polyester graft. Graft size is chosen based on the diameters of the celiac artery and SMA. I believe the graft limbs should be larger than 6 mm, because of the early and late failures we have seen from intimal hyperplasia and pseudointima in this smaller-sized limb. An end-to-side anastomosis is done to the aorta with running polyproprolene suture. In some patients, a parachute technique helps. The anastomosis is tested by infusing saline into the graft with clamps still in place. Blood flow is restored slowly through the native aorta after backbleeding and fore-bleeding through the limbs has been done. The limbs of the graft are clamped at their origins. Acute kidney injury and distal embolization rarely occur when the supraceliac aorta is free of disease and the ischemia time is within the aforementioned range. Additional mannitol and Lasix can be given to stimulate urine output as needed after restoration of aortic blood flow or if the ischemia time exceeds 20 min. We prefer prosthetic over vein in this position, because the vein tends to be pulled into the aorta at the proximal anastomosis, which leads to stenosis and eventual failure.