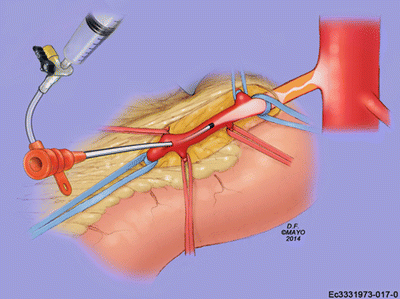
Fig. 17.1
Superior mesenteric artery exposed with proximal and distal control (By permission of Mayo Foundation for Medical Education and Research. All rights reserved)
Exposure and Control of the Supraceliac Aorta
In some cases it may be necessary to obtain some level of proximal control at the level of the supraceliac aorta. This should generally be avoided if possible, but when necessary it should be performed through a midline laparotomy and transperitoneal approach to allow for evaluation of the bowel at the same time. First, pack the abdominal contents inferiorly, and retract the left lobe of the liver superiorly and to the patient’s right. To increase exposure it might be helpful to divide the left triangular ligament. Next, enter the lesser sac by incising the gastrohepatic ligament to the right of the esophagus. When extending this incision, be aware that a replaced or accessory left hepatic artery may be present, and care should be taken to avoid injury to this vessel. The esophagus and stomach are then retracted to the left to expose the right crus of the diaphragm. Incise the posterior peritoneum, and separate the right crus to expose the anterior supraceliac aorta. Clear the walls of both sides of the aorta with blunt finger dissection. To complete the exposure perform a vertical incision extending cephalad into the thoracic cavity, through the median arcuate ligament and the right crus, anterior to the anterior aorta. Once there is adequate clearance both medially and laterally, a curved aortic clamp can be placed.
Thromboendarterectomy
After proximal and distal control is gained, a longitudinal arteriotomy is made on the anterior wall of the SMA. The endarterectomy is begun with a plaque elevator. The optimal endarterectomy plane is that between the inner and outer medial layers. The proximal endpoint is obtained by endarterectomy of the plaque as close to the origin of the SMA as possible. In most cases, atherosclerotic disease involves the ostia, and it can be very difficult if not impossible to adequately perform an endarterectomy of the origin from this location. This needs to be considered if an endarterectomy is intended to be the sole procedure as it will be prone to failure and should include either retrograde stenting or bypass if the origin has significant involvement.
The plaque is then elevated under full vision while the endarterectomy is continued distally. When branches of the SMA are encountered, eversion endarterectomy of those branches is performed. At the distal arteriotomy, the plaque is either feathered so that a smooth taper is achieved in transition to normal distal intima or sharply transitioned and tacked down with Prolene suture. After completion of the endarterectomy, all residual debris and medial fibers are excised because of their potential contribution to embolization or hyperplastic restenosis. The endarterectomy surface is irrigated with heparinized saline to facilitate visualization and removal of all debris.
The management of acute SMA thrombosis caused by underlying atherosclerotic lesions with simple surgical thrombectomy is unlikely to be durable [10]; however, in some selected high-risk patients that would not tolerate a long revascularization procedure, it may be the best option.
If no bypass is planned, the arteriotomy should be closed with a patch. The patch may be constructed from a piece of vein or synthetic material. If there is significant peritoneal soilage, a vein patch is preferred. The patch should be cut to the appropriate size. The ends should be rounded, to avoid narrowing. Next a double-armed, nonabsorbable monofilament suture is used for the repair. The needle is passed from inside of the artery to outside, through all layers of the wall, to avoid creating an intimal flap. Starting at one end, pass one needle through the patch and through the artery and secure with a knot on the outside of the artery. Suturing is continued around the artery, starting at the far wall, with the needle passing outside-in on the graft and inside-out on the artery. Use an assistant to follow, making sure to keep appropriate tension on the suture line. Handle the artery with care, especially the intima. This reduces the risk of late thrombosis. When halfway around with each needle, repeat the process at the opposite apex with another double-ended suture, meeting in the middle. Before closing the arteriotomy, inflow and outflow are released to remove any clot, and the artery is flushed with heparinized saline. The sutures are then tied on the sides of the repair.
Superior Mesenteric Artery Bypass
Patients with SMA thrombosis will typically have severe atherosclerotic plaque at the orifice of the SMA. Those patients who are identified early and have no or limited intestinal necrosis may undergo SMA bypass grafting with a prosthetic conduit. Some of these patients may have fluid within the peritoneal cavity. This finding is not necessarily a contraindication to the use of a prosthetic graft. However, if the patient has perforation with significant spillage, use of autologous vein as conduit is preferred. A good-quality vein is mandatory; if the saphenous vein is inadequate, the femoral vein may be used instead [11].
These patients are often critically ill, so it is imperative to perform the operation rapidly and efficiently. In the acute setting, bypass to the SMA alone (single-vessel bypass) is strongly preferred [12, 13]. Inflow for mesenteric bypass grafts may be derived either above or below the renal arteries. The graft is antegrade if it originates cephalad to the celiac artery. The graft is retrograde if it originates from the infrarenal aorta or a common iliac artery. An antegrade bypass has definite advantages, mainly improved hemodynamics, and also a straighter graft configuration that minimizes graft kinking. Typically, there is also reduced atherosclerotic calcification in the supraceliac aorta [14]. One of the main disadvantages of an antegrade bypass is the need to clamp the supraceliac aorta for the proximal anastomosis. Use of side-biting clamps may be possible but not always practical. Clamping the supraceliac aorta carries an increased risk of cardiac events, embolization, and ischemia. It is essential to ensure that the supraceliac aorta can safely be clamped, prior to proceeding with an antegrade bypass. It should be acknowledged that reoperation and attempts to reexpose the supraceliac aorta are much more difficult the second time around and generally riskier.
Antegrade Mesenteric Bypass
Supraceliac aorta–superior mesenteric artery bypass is performed through a midline incision and transperitoneal approach. Begin the dissection by dividing the gastrohepatic ligament and retracting of the left lobe of the liver to the right. This is followed by incision of the diaphragmatic crus and exposure of the anterior aspect of the aorta. For more details see section on Exposure and Control of the Supraceliac Aorta. In most cases Dacron grafts or reinforced expanded polytetrafluoroethylene (ePTFE) is preferred. If the use of a prosthetic graft is combined with resection of necrotic bowel, Dacron should be used, and it should be soaked in rifampin. Soak the graft for 15 min in a 1 mg/ml saline solution of rifampin at 37 °C [15] prior to implantation. After implantation the graft should be excluded with the omentum prior to performing enterectomy [5]. Autologous vein grafts are usually reserved for severely contaminated cases. The femoral vein is an excellent conduit for mesenteric arterial bypass [11]. The SMA is typically the mesenteric vessel involved; however, if the celiac artery needs to be revascularized, consider an end-to-side anastomosis to the aorta, followed by an end-to-side distal anastomosis to the common hepatic artery. When revascularizing the SMA, it is usually necessary to tunnel the graft behind the pancreas. Use caution when creating the retropancreatic tunnel. If the area appears narrow or is scarred from previous pancreatic inflammation, consider a tunnel anterior to the pancreas to ensure that it is not compressed and to avoid causing bleeding from disrupted pancreatic veins [16]. When an anterior tunnel is needed, consider using vein conduit because the graft will lie posterior to the stomach. After creation of the tunnel, either anterior or posterior, perform an end-to-side anastomosis to the SMA at that level.
Retrograde Mesenteric Bypass
There are several combinations of graft orientation that may be used for a retrograde bypass, and there are several types of conduit to choose from. The choice of orientation and conduit is largely influenced by the adequacy of the potential inflow vessels and by the presence or absence of peritoneal soilage. Foley et al. have reported the use of the distal infrarenal aorta or the infrarenal aorta–right common iliac artery junction as the preferred sites for the inflow anastomosis. There does not appear to be a significant difference in long-term patency between antegrade and retrograde bypasses (primary patency of 93 % and 95 % at 36 months, respectively) [17]. An advantage to the retrograde bypass is that most surgeons are more comfortable with the approach to the infrarenal aorta. Another advantage is that dissection and clamping of the infrarenal aorta carries less physiologic risk than dissection and clamping of the supraceliac aorta. The main disadvantage is the potential for graft kinking. Kinking is less likely to occur if Dacron or reinforced ePTFE is used and if the right common iliac is used as the site for distal anastomosis. The key to avoiding graft elongation, angulation, or kinking of the graft is to cut it to length with the SMA in a nearly anatomic position [13].
Our preference for retrograde graft orientation is the “reverse C-loop” configuration, with its origin from the right common iliac artery (Fig. 17.2). This way aortic clamping is avoided. It also provides a good lie for the graft and theoretically decreases the chance of it kinking. Similar C-loop grafts can be used for other inflow sources, such as the left iliac or distal infrarenal aorta if the right common iliac artery is not adequate. The lie of C-loop grafts tends to be improved by slightly increasing graft length and by performing an end-to-end anastomosis with the SMA. If none of those inflow vessels are suitable for bypass, a very short retrograde bypass using a larger diameter graft (8–10 mm) can be performed from the proximal infrarenal aorta. Keep in mind that there is a higher potential for graft kinking with shorter grafts.

Fig. 17.2
Superior mesenteric artery bypass with a synthetic graft in a “reverse C-loop” configuration
The SMA and distal aorta are dissected out and isolated in a similar manner to the techniques described earlier. In addition, the fourth portion of the duodenum needs to be fully mobilized for a more lateral approach to the SMA to facilitate the retrograde bypass. The retroperitoneum is divided distally along the aorta to a point just beyond the level of the aortic bifurcation. Assess both the distal aorta and common iliac arteries to determine the optimal inflow vessel for the proximal anastomosis. Typically, grafts made of Dacron or of ringed, reinforced ePTFE are the preferred conduits. The use of autologous vein has the potential to cause kinking when the viscera are replaced. However, if there is significant peritoneal contamination, consideration must be made to their use. When a retrograde vein bypass is performed, the graft may be brought straight up from the right iliac artery so that it lies between the aorta and the duodenum and then anastomosed to the posteromedial wall of the SMA. This is done to decrease the chance of kinking. Care must then be taken to check for kinking after the viscera have been repositioned.
The junction of the aorta with the right common iliac artery is the preferred site for the proximal anastomosis. Grafts originating from the midportion of the infrarenal aorta are prone to kinking when the viscera are returned to their normal position. The graft to the SMA is passed cephalad, turned anteriorly and inferiorly 180°, and anastomosed to the anterior wall of the SMA just beyond the inferior border of the pancreas [16]. If performed correctly, a gentle “C-loop” should be formed by the graft, and this will decrease the chance the graft will kink when the viscera are restored to their anatomic position after retractor removal. The ligament of Treitz and peritoneum are closed over the graft to exclude it from the peritoneal cavity.
Hybrid Technique: Retrograde Open Mesenteric Stenting (ROMS)
Milner et al. [18] reported the first technically successful ROMS, a hybrid approach for the treatment of acute atherosclerotic SMA thrombosis that involves a less invasive mesenteric revascularization without compromising important general surgical principles. They experienced no ROMS-related complications or morbidity. Wyers [19] and coauthors from Dartmouth reported similar findings in their report on 6 patients. In a well-stocked operating suite, with readily available fluoroscopy, ROMS should take less time than a saphenous vein harvest and bypass procedure and is probably quicker than synthetic bypass as well. It maintains sound surgical principles of thorough abdominal exploration while minimizing physiologic stress and may prevent a prosthetic graft infection in the face of bowel perforation or resection. In addition, a patient with mesenteric ischemia likely has aortoiliac disease compromising inflow into the bypass graft. Cross-clamping of an aorta that is heavily diseased and suturing to a heavily diseased graft donor vessel expose the hypotensive and acidotic patient to hazardous changes in hemodynamics and potential injury to atherosclerotic and calcified vessels. All of these potential problems with mesenteric bypass are avoided with ROMS. If the endovascular procedure proves difficult or impossible, converting to a bypass operation is easy with the exposure being half complete.
Our technique and early experience were previously reported by our group in 2010 [20]. After exposing the SMA, the infrapancreatic SMA is isolated and punctured with either a micropuncture needle or an 18-gauge needle. Then, over a wire, a 6 or 7-French sheath is placed. A local thromboendarterectomy through a longitudinal arteriotomy and placement of a patch angioplasty, as previously described, may be necessary prior to placement of wire and sheath. In some instances a transverse arteriotomy and embolectomy with an embolectomy catheter (4 Fr proximally and 3 Fr distally) may be needed to thrombectomize the proximal SMA prior to placement of a wire and sheath. After placement of a wire and sheath, a retrograde angiogram is performed to confirm the SMA occlusion proximally (Fig. 17.3). Appropriate wire and catheter selection is important when attempting to cross these lesions. We prefer a Berenstein catheter (Cordis, Inc, Warren, NJ) and soft-angled glidewire (Terumo Interventional Systems, Somerset, NJ) to maneuver through the occlusion. Once wire and catheter have crossed the lesion, an aortogram is performed (Fig. 17.4). If there is any question as to appropriate sizing for the mesenteric vessel, intravascular ultrasound is a valuable tool. The wire is then exchanged for a lower profile 0.018- or 0.014-in. platform. The lesion, if necessary, can then be predilated with a 3- or 4-mm angioplasty balloon. A 6–8-mm balloon expandable stent should be deployed partially into the aorta and flared (Fig. 17.5). More than one stent may be required to cross the entire length of the lesion. Completion angiography in the anteroposterior and lateral projections with or without pressure measurements is performed across the lesion to confirm patency. The arteriotomy is closed with a patch angioplasty with nonabsorbable monofilament sutures (Fig. 17.6). Small bowel viability is reassessed at the end of the operation.