Fig. 13.1
Treatment trends in patients with chronic mesenteric ischemia treated by open or endovascular revascularization at the Mayo Clinic
In most centers, including ours, mesenteric angioplasty and stenting is currently the first choice of treatment in patients with CMI who have suitable lesions, independent of their clinical risk. A careful review of pre-procedure CTA with attention to anatomical factors determines selection of open or endovascular approach. The SMA is the primary target for revascularization, and as such the anatomy of the SMA is the most important determinant of choice of therapy. The ideal lesion for angioplasty and stenting is a short, focal stenosis or occlusion with minimal to moderate calcification or thrombus (Fig. 13.2). For celiac axis (CA) lesions, angioplasty and stenting carries higher rates of restenosis [21], and should not be performed if there is active compression by the median arcuate ligament, unless this has been surgically released. We found no benefit for two-vessel stenting [21]. The technical difficulty of endovascular procedures is increased by presence of severe eccentric calcification, flush occlusion, and in patients with longer lesions, small vessels, and tandem lesions affecting branches. Although these anatomical features do not contraindicate the use of stents, technical result is often not optimal with higher rates of arterial complications (e.g., distal embolization, dissection) and restenosis [22, 23]. Our preference in lower-risk group has been to offer open revascularization if the anatomy is unfavorable for angioplasty and stenting [24, 25]. Mesenteric bypass has also been increasingly performed in patients who have failed a percutaneous intervention or in those with multiple recurrences or other non-atherosclerotic lesions, such as vasculitides and neurofibromatosis. Finally, for patients who are not good candidates for open repair because of severe comorbidities or cachexia, stenting can be used as a “bridge” to open surgical bypass, or endovascular recanalization can be attempted to treat complex lesions.
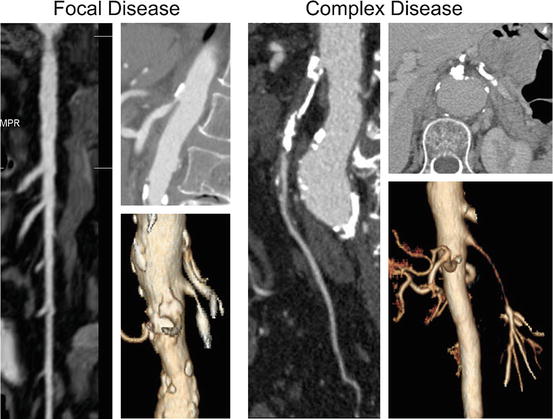

Fig. 13.2
Computed tomography angiography is the most useful imaging study to plan revascularization. Anatomical characteristics of the superior mesenteric artery (SMA) can be used to identify patients with focal disease where angioplasty and stenting is favored or patients with complex disease where endovascular therapy is technically more challenging. Lesions with unfavorable anatomy for stenting include heavily calcified occlusions, long-segment occlusions, and long-segment stenosis involving multiple branches
Pre-procedure Evaluation
Endovascular mesenteric revascularization carries definitive risk. The average 30-day mortality in a recent systematic review was 6 % (0–21 %), surpassing the mortality reported for other types of endovascular interventions, including aortic, renal, and carotid procedures. Even though most interventions are done using local anesthesia, these patients typically undergo a comprehensive medical evaluation to identify and optimize cardiovascular risk factors and their nutritional status. Many of the comorbidities may require medical therapy to be started prior or after the intervention, depending on its severity. Revascularization should not be excessively delayed. Patients who present with deterioration of symptoms should be admitted, started on intravenous heparin, and treated urgently within 24–48 h. Patients with iodinated contrast allergy should be premedicated with steroid preparation. Those with chronic kidney disease who have serum creatinine level >1.5–2.0 mg/dL (133–177 Mmol/L) undergo intravenous hydration with sodium bicarbonate and oral acetylcysteine, starting the day prior to intervention. Review of pre-procedure imaging (CTA, MRA, or conventional angiography) is key to select the ideal approach based on the angle of origin of the mesenteric vessels in relation to the aortic axis, the amount of calcium and thrombus load, and the presence of important collaterals or unusual anatomy (e.g., replaced hepatic) in proximity to the target lesion.
Selection of Access Site
The choice of ideal access is dependent on vessel anatomy, extent of occlusive disease, angle of origin from the aorta, and physician preference. The advantages of femoral approach include the frequent use and familiarity, ability to use shorter delivery system, and most importantly the exceedingly low rate of access site complications. However, because the CA and superior mesenteric artery (SMA) have acute angle of origin from the aorta, mesenteric interventions can be difficult to perform via the femoral approach. Excessive angulation often results in added time and radiation exposure, multiple guidewire exchanges, and in some cases, treatment failure.
Therefore, the ideal approach remains controversial. The author’s preference is to use the left brachial artery access with a small 1–2 cm incision to surgically expose the artery. This is done under local anesthesia. Brachial access is particularly beneficial in the patient with difficult anatomy, such as those with flush occlusions, acute aortic angles, or long-segment lesions affected by very high-grade stenosis. Although the use of brachial access has been associated with higher rate of local complications when done totally percutaneously, it may reduce rate of severe mesenteric artery complications including dissections, vessel perforations, and embolization. Finally, we have increasingly utilized the radial artery access, but this is limited by need for longer delivery system and smaller profile sheath and devices.
Diagnostic Mesenteric Angiography
Diagnostic angiography has been discussed in detail in Chap. 2 (Fig. 13.3). Diagnostic angiography is most often done immediately prior to a planned intervention, either through the femoral or brachial approach. Access is established using ultrasound guidance and 0.035 in guidewire system. A 5 Fr sheath is positioned in the external iliac artery, and 5 Fr diagnostic flush catheter is advanced to T12 level over a 0.035 in guidewire. Modest intravenous heparinization (40 units/kg) is recommended prior to selective catheterization of the mesenteric arteries. Low-osmolar contrast agent (e.g., Visipaque®) minimizes abdominal discomfort during selective injections. Choice of catheter shape is dependent upon access site, angle of origin, and individual preference. MPA catheter is ideal for selective catheterization via brachial approach, whereas a secondary curve catheter (e.g., SOS or Simmons) or a catheter with more acute curve (e.g., Cobra 2) can be used for interventions done via femoral approach (Fig. 13.4). A complete study includes abdominal aortogram with anterior-posterior and lateral views to define the location, severity, and extent of visceral artery involvement and to identify concomitant lesions in the aorta, renal, or iliac arteries. The optimal projection to display the proximal CA and SMA is a lateral view, and for the origin of the IMA, it is a 15° right lateral-oblique view. Selective angiography is necessary to confirm the severity of disease and to identify tandem lesions and collateral patterns. In patients with questionable lesions, pressure gradients can be measured using pressure wire, “pull-back,” or simultaneous pressure measurement technique [26].
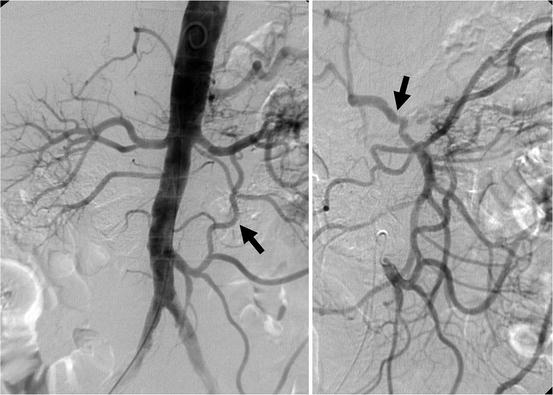
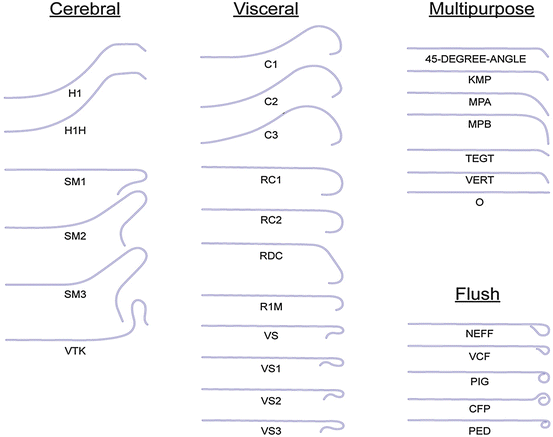

Fig. 13.3
Abdominal aortogram with right anterior-oblique view demonstrates large patent inferior mesenteric artery (IMA). Selective IMA angiography confirms collateralization to the superior mesenteric artery via arc of Riolan (arrow) and collateralization to the celiac axis via gastroduodenal artery (arrow)

Fig. 13.4
Catheter shapes frequently utilized for mesenteric interventions
Selective Catheterization
Selective catheterization requires systemic heparinization (80 mg/kg), which should be administered prior to catheter manipulations in order to achieve an activated clotting time over 250 s. Using the brachial artery access, a 6 or 7 Fr 90 cm hydrophilic sheath is positioned in the descending thoracic aorta above the CA origin. A 5 Fr MPA catheter is ideal for selective catheterization of the mesenteric arteries using the brachial approach, whereas a SOS or VS1 catheter can be used from the femoral approach. The initial selective angiography should demonstrate the origin of the vessel from the aortic wall and the severity of the stenosis and should document the distal branches for comparison with post-intervention views.
The target lesion is initially crossed using a 0.035 in soft angled glidewire, which is exchanged for the interventional wire of choice after confirmation of true lumen access. The authors’ preference is to use a small profile (0.014 or 0.018 in) stiff guidewire for most interventions. The tip of the guidewire should be visualized and positioned within the main trunk of the SMA, rather than within small jejunal branches, which are prone to perforate or dissect.
Embolic Protection Devices
The use of embolic protection devices is highly controversial. The potential advantage is limiting or preventing distal embolization, which has been described as a known intraprocedural complication and cause of death after mesenteric interventions. Limitations include the added cost and the potential risk of arterial damage by the filter basket. In addition, there is limited information on which is the ideal filter device and where it should be placed within the mesenteric vessel. The Mayo Clinic group has reviewed the anatomy of the SMA to identify diameter and number of jejunal branches. Most patients have an average of 13 major jejunal branches, and >90 % of these branches originate >5 cm distal to the aortic origin of the SMA. The average diameter of the SMA in this location is 5–6 mm. Therefore, the most frequently utilized filter device would be a 7-mm filter basket positioned approximately 5 cm distal to the SMA origin.
In our experience, embolic protection devices are used selectively. Patients with acute or subacute mesenteric ischemia have a component of fresh in situ thrombosis and may be more prone to distal embolization. Among patients with chronic lesions, we found higher embolization rates in those with occlusions, severe calcification, and lesions longer than 30 mm. The author recommends use of embolic protection in these situations [22]. Our preference is to use a 320 cm working length 0.014 in filter wire (Spider RX, Covidien, Plymouth, MN; Fig. 13.5). Alternatively, Brown and associates described the use of temporary balloon occlusion and aspiration with GuardWire (Medtronic, Minneapolis, MN) [27]. If a 0.035 in stent is selected, a two-wire technique can be used by combining a 0.014 in filter wire with a 0.018 in “buddy wire.” The stent is introduced via both wires for better support and to facilitate subsequent retrieval of the embolic protection device.
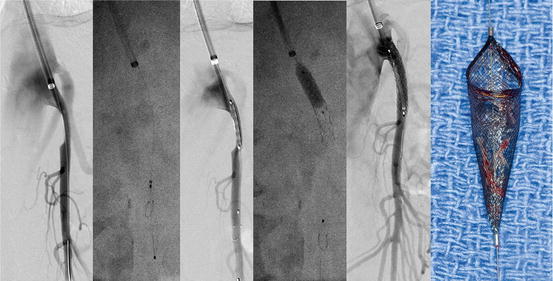

Fig. 13.5
Angioplasty and stenting of a focal stenosis of the superior mesenteric artery (SMA) stenosis using brachial approach. After selective angiography, the lesion is crossed, and a 0.014 in Spider Rx filter wire (Covidien, Plymouth MN) is deployed in the main trunk of the SMA, avoiding jejunal branches. The entire lesion is treated by balloon-expandable stent, which is extended 1–2 mm into the aorta and flared proximally. Completion angiography demonstrates patency of the stent without embolization or dissection. Appearance of a filter basked with moderate amount of debris
Choice of Stent
The vast majority of patients (>90 %) are treated using balloon-expandable stents. These have the advantages of wide range of length, precise deployment, and radial force. Because mesenteric lesions mimic renal artery lesions and are ostial and highly calcified, precise deployment and radial force are major advantages. Nonetheless, longer lesions affecting the bend of the SMA or those in tortuous segments may not be ideally suited for balloon-expandable stents. In these cases a short self-expandable stent may be used. If the lesion is long, it is not uncommon to combine a balloon-expandable stent for the ostia of the lesion with a self-expandable stent distally. Finally, we have recently shifted our practice to use preferentially covered balloon-expandable stents, which are resistant to intimal hyperplasia and have higher patency rates compared to bare metal stents. Covered stents are avoided in segments with critical side branches, which need to be preserved.
Angioplasty and Stenting of Mesenteric Stenosis
The primary goal of percutaneous treatment is to restore antegrade flow into at least one of the three mesenteric arteries, preferentially the SMA. First reports described successful results with balloon angioplasty alone, but elastic recoil and restenosis have limited its utility when used for ostial lesions [18, 28–37]. Although there are no prospective comparisons between angioplasty alone and primary stenting, most agree that routine stenting is indicated given that mesenteric lesions resemble renal artery stenoses [21, 27, 38–51]. Although there are no randomized comparisons between SMA and celiac stent placement, retrospective studies suggest that celiac stenting is associated with more recurrences in the first year after treatment [21]. In patients with compression of the CA by the median arcuate ligament, there is risk of stent fracture and compression. The role of two-vessel stenting remains controversial. Two retrospective studies by the MGH group and by Silva and colleagues have shown a nonsignificant trend towards less recurrence with two-vessel stenting [43, 46]. Malgor and colleagues from the Mayo Clinic reported nearly identical recurrence rates at 2 years in patients treated by SMA stents (78 %) compared to two-vessel stenting of the SMA and CA (60 %). Two-vessel mesenteric interventions may have a role in select patients with severe gastric ischemia and who do not have good collateral network between the CA and SMA. However, there is no proven benefit that routine two-vessel stenting provides more durable relief, and a second intervention adds cost and potential risk of complications.
CA intervention may be considered in higher-risk patient who fails attempted recanalization of the SMA or in those where an SMA intervention is felt to have a low yield for success due to excessive calcification or long-segment occlusion. In these patients, celiac stenting may be considered a “bridge” to open bypass or retrograde SMA stenting [52]. Angioplasty of the IMA in our experience carries a higher risk of rupture, dissection, or embolization, and is not advised with rare exceptions.
A brachial artery approach is preferred for patients with very angulated origin of the aorta and in those with occlusions or longer lesions. The author’s preference is to use brachial artery approach whenever possible (Fig. 13.5). This offers excellent support with small profile system and precise stent deployment in patients with acute SMA angle. Although the risk of puncture-related complications is higher using total percutaneous technique, one option is to use a small 1–2 cm incision under local anesthesia to expose and repair of the brachial artery; less frequently, radial approach has been used.
Percutaneous access is established with a 0.018 in micropuncture set using ultrasound guidance, after which the system is exchanged to 0.035 in. Full systemic heparinization (80 mg/kg) is administered prior to catheter manipulations to achieve an activated clotting time of >250 s. A 6 or 7 Fr 90 cm hydrophilic sheath is positioned in the descending thoracic aorta above the CA origin. A 5 Fr MPA catheter is ideal for selective catheterization of the mesenteric arteries using the brachial approach, whereas a SOS or VS1 catheter can be used from the femoral approach. The initial selective angiography should demonstrate the origin of the vessel from the aortic wall and the severity of the stenosis and should document the distal branches for comparison with post-intervention views. The target lesion is initially crossed using a 0.035 in soft angled glidewire, which is exchanged for the interventional wire of choice after confirmation of true lumen access. The author’s preference is to use a small profile (0.014 or 0.018 in) stiff guidewire for most interventions. Most recently, our practice has changed to covered stents, based on a recent report which indicates superior patency rates compared to bare metal stents [53]. The tip of the guidewire should be visualized and positioned within the main trunk of the SMA, rather than within small jejunal branches, which are prone to perforate or dissect (Fig. 13.6). Embolic protection may be useful in select patients with occlusions, long lesions (>30 mm length), severe calcification, thrombus, and acute or subacute symptoms; the author’s preference is to use a 320 cm working length 0.014 in filter wire (Spider RX, Covidien, Plymouth MN). Alternatively, Brown and associates described the use of temporary balloon occlusion and aspiration with GuardWire (Medtronic, Minneapolis, MN) [27]. If a 0.035 in stent is selected, a two-wire technique can be used by combining a 0.014 in filter wire with a 0.018 in “buddy wire”; the stent is introduced via both wires for better support and to facilitate subsequent retrieval of the embolic protection device (Fig. 13.5). Pre-dilatation is recommended for tight stenosis, occlusions, severe calcification, and to size stents. A balloon-expandable stent with diameters ranging from 5 to 8 mm is used in >95 % of cases, allowing precise deployment and greater radial force. The stent is positioned under protection of the sheath, covering slightly more than the entire length of the lesion. Positioning the stent in the aortic lumen is critical to avoid missing the proximal portion of the lesion. It is important to position the stent 1–2 mm into the aortic lumen (Fig. 13.5). Ideally, the stent should be flared gently into the aorta, which prevents missing the ostia and facilitates re-catheterization if needed. Occasionally a self-expandable stent is needed to treat a non-ostial lesion or segments with excessive tortuosity, extending beyond the angulated portion of the SMA.
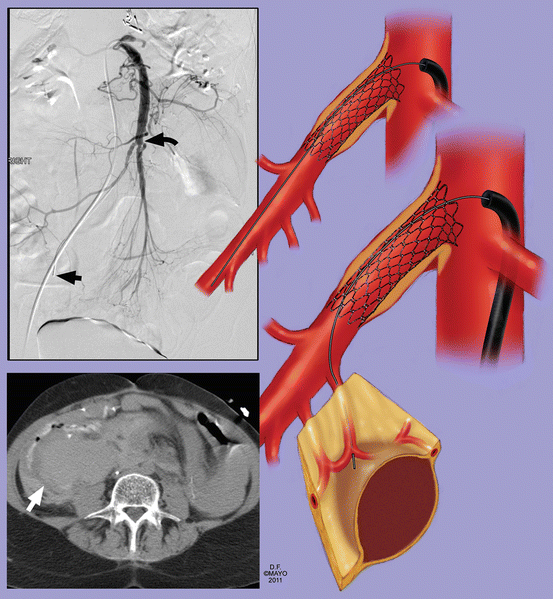

Fig. 13.6
An important technical point is to visualize the tip of the guidewire during the intervention and to position the guidewire in the main trunk of the superior mesenteric artery (curved black arrow) as opposed to distal jejunal branches (black straight arrow) which are prone to perforation resulting in mesenteric hematoma (white straight arrow) (By permission of Mayo Foundation for Medical Education and Research. All rights reserved)
Specific Situations
Recanalization of Mesenteric Occlusions
The technique is slightly modified in patients with difficult occlusions. In these cases it is of paramount importance to use the brachial approach and a stiff support system, which is accomplished by combining a 7 Fr sheath, 7 Fr MPA guide catheter, and 5 Fr MPA catheter (Fig. 13.7). In the author’s opinion attempting a difficult recanalization from the femoral approach adds time, contrast, and catheter manipulations and is fraught with exceedingly high failure rates. Ideally the tip of the MPA catheter is used to engage the stump of the occluded SMA (Fig. 13.8), and sufficient support is provided by the combination of the sheath and guide catheter. The lesion is crossed using a straight tip, hydrophilic, soft 0.035 in glidewire but also using 0.018 in or 0.014 in guidewires if needed. It is ideal to avoid the subintimal plane, which is best achieved by using straight tip guidewires. A Quick-Cross (Spectranetics, Colorado Springs CO) or an alternative support catheter or even a small coronary balloon may be needed to cross a tight lesion. Once the lesion is crossed, access into the true lumen should be confirmed. Our preference has been to use embolic protection device (e.g., Spider RX, Covidien, Plymouth, MN) with two-wire technique routinely in cases of total occlusion.
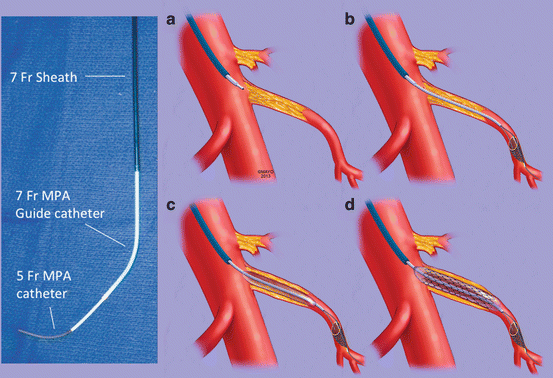

Fig. 13.7




Technique of recanalization and primary stenting of a total superior mesenteric artery (SMA) occlusion. In these cases, a stiff support system is build with combination of a 7 Fr 90 cm hydrophilic sheath, 7 Fr 100 cm MPA guide catheter and 5 Fr 125 cm MPA catheter. The stump of the occluded SMA is engaged by the sheath–catheter combination (a); the lesion is crossed using a straight glidewire. After true lumen access is confirmed, a 0.014 in filter wire and a 0.018 in buddy wire are deployed into SMA via 0.035 in catheter (b); the lesion is pre-dilated (c) and stented using a balloon-expandable stent (d) (By permission of Mayo Foundation for Medical Education and Research. All rights reserved)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


