Tracheal measurement
Males (n = 40)
Females (n = 20)
All (n = 60)
Males versus females statistics
Length (mm)
Difference between means 6.8
Mean ± standard deviation
105.1 ± 9.8
98.3 ± 8.7
102.8 ± 9.9
95% CI 1.6–11.9
Range
86.1–123.9
78.8–123.0
78.8–123.9
P = 0.01
Maximum antero-posterior diameter (mm)
Difference between means 3.4
Mean ± standard deviation
22.6 ± 2.9
19.2 ± 2.6
21.4 ± 3.2
95% CI 1.9–4.9
Range
16.8–28.6
12.7–23.8
12.7–28.6
P < 0.0001
Maximum transverse diameter (mm)
Difference between means 4.2
Mean ± standard deviation
27.1 ± 3.4
22.9 ± 2.6
25.7 ± 3.7
95% CI 2.5–5.9
Range
20.1–34.5
17.3–27.8
17.3–34.5
P < 0.0001
Estimated volume (cm3)
Difference between means 10.8
Mean ± standard deviation
35.6 ± 6.8
24.7 ± 6.1
32.0 ± 8.3
95% CI 7.2–14.4
Range
18.9–53.6
12.9–35.7
12.9–53.6
P < 0.0001
Subcarinal angle of bifurcation (°)
Mean ± standard deviation
76 ± 20
81 ± 20
78 ± 20
95% CI −15.2 to 6.3
Range
36–121
47–115
36–121
P = 0.41
Airway Assessment, Grading Systems, and Symptoms of Airway Stenosis
It is important to ascertain the level of the stenosis within the airway, whether it is a solitary lesion or multifocal, the length of the stenosis and how narrow it becomes. Computed tomography is the mainstay of imaging to visualize the upper airway and offers the surgeon a “road map” to assess the entire upper airway. Specifically, CT allows for objective and quantifiable assessment of the length and diameter of the stenosis and the normal caliber of the airway. CT data can be reformatted into coronal sections and 3-D reconstruction (Fig. 48.1) of the airway to reveal a cast of the airway to aid in the visualization of the narrowing and its relationship with surrounding structures. Due to the relatively long acquisition times, MRI images can be blurry due to the movement of the trachea from breathing and the beating of the heart. Because of this, MRI is not as well suited to visualizing the airway compared to CT.
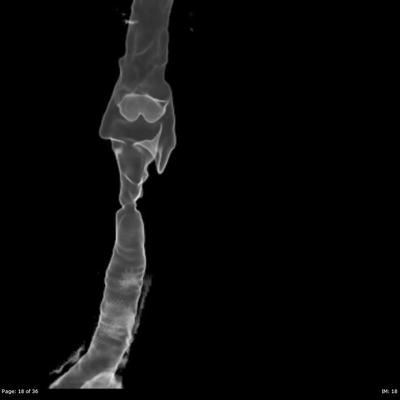

Fig. 48.1
3-D reconstruction of airway. Axial CT data is carefully reconstructed into this three-dimensional image that can be rotated and manipulated. The area of stenosis can be clearly defined in this image
The gold standard in evaluating laryngotracheal stenosis continues to be direct visualization in the operating room under general anesthesia. Assuming the patient is able to breathe on his/her own prior to surgery, the patient can be given oxygen by mask while using IV agents to achieve anesthesia. The airway can be controlled using a laryngeal mask airway if difficulties arise so as not to disturb the laryngeal or tracheal airway. The larynx is then suspended to allow bimanual examination and manipulation of the airway. Jet ventilation is the preferred method because it does not cannulate the airway leading to artificial changes one can get with intubation (laryngeal or tracheal injuries with bleeding, dilation of the stenosis leading to artificially enlarged stenoses). If endotracheal intubation is performed, the endotracheal tube is removed each time the airway is to be examined and promptly replaced as the oxygen saturation begins to decline.
A 0-degree Hopkins rod is introduced down the barrel of the laryngoscope and is used to visualize the airway lumen via a video monitor. The video system allows the members of the airway team to see the endoscopic examination in real time and captures video for review after the procedure (Fig. 48.2). Structures and landmarks can be measured using the Hopkins rod by marking the barrel of the Hopkins rod with tape at the upper incisors when the tip is at the level of the glottis, lower border of the cricoid, and the upper and lower parts of the stenosis. This allows for accurate measurements of the length of stenosis that can be correlated with the CT data. The amount of narrowing within the airway can be described using several popular grading systems such as the Cotton-Myer or McCaffrey (Tables 48.2 and 48.3) that assess the percentage of the airway that remains compared to the normal caliber. These systems lack accuracy, lumping patients with a broad range of stenoses into the same category (as in grade I Myer-Cotton with a 0–50% narrowing) because functionally they behave similarly. The size of stenosis can be accurately measured by introducing instruments of known diameter and passing them through the area of stenosis.
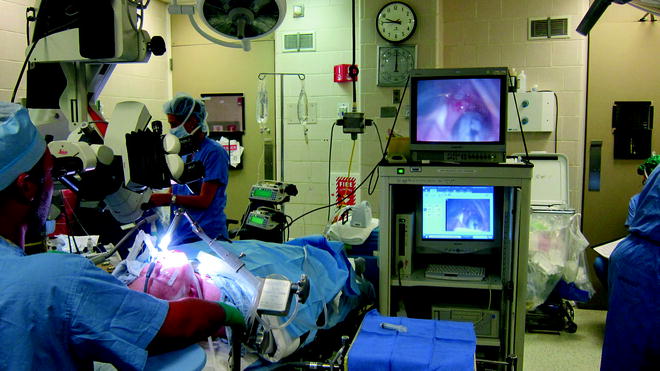

Fig. 48.2
Videoendoscopic tower. The videoendoscopic tower allows the surgical team to visualize the stenosis and capture video and still images that can be reviewed in the OR or clinic to facilitate patient education, assess response to intervention, or plan future interventions
Table 48.2
Myer–Cotton grading scale
Grade I | Up to 50% obstruction |
Grade II | 51–70% obstruction |
Grade III | Above 70% with any detectable lumen |
Grade IV | No lumen |
Table 48.3
McCaffrey classification
Stage I | Lesions confined to the subglottis or trachea and are less than 1 cm long |
Stage II | Lesions are isolated to the subglottis and are greater than 1 cm long |
Stage III | Subglottic/tracheal lesions not involving the glottis |
Stage IV | Lesions involve the glottis |
The airway can be assessed in the awake-clinic setting with flexible nasolaryngoscopes or bronchoscopes to immediately gain an understanding of the magnitude of the airway stenosis. This is ideal for patients who wish to avoid a diagnostic procedure in the operating room or for those who are too medically unstable to safely undergo a diagnostic procedure under general anesthesia. This method requires only topical application of a local anesthetic with a video system to visualize and record the examination. Our preferred method is to spray 2% lidocaine with 0.025% oxymetazoline into the nasal cavities bilaterally followed by a transtracheal injection of 2 cc of 4% lidocaine. The flexible scope is introduced down to the level of the mainstem bronchi/carina and pulled back out of the airway. In a fashion similar to that previously described for marking distances using a rigid Hopkins rod, starting from distal to proximal, the flexible scope can be marked to represent the distal and proximal edges of the stenosis and the level of the vocal folds.
The biggest advantage of direct airway visualization is the ability to palpate and manipulate the airway and alter the stenosis using rigid instruments, balloons, lasers, or medications that can be applied or injected at the time of the endoscopy to alleviate the effects of the stenosis. We will discuss the specifics of these treatments later in this chapter.
The most common symptoms of upper airway stenosis, including dysphonia, dyspnea, cough, and stridor, occur after stenosis reaches a critical narrowing to induce these symptoms. At the point of narrowing in the airway, the resistance to airflow is inversely proportional to the fourth power of the radius at the stenosis and directly proportional to the length of the stenosis. As the resistance increases, the patient has to work harder to move air through the constricted segment giving the patient dyspnea that begins with heavy activity (running, swimming, climbing) and progresses to dyspnea at rest in highly critical stenosis. Turbulent airflow leads to the airway noise we call stridor and alters the way the air hits the vocal folds, leading to a weak and hoarse voice. The brassy cough is due to the altered mucociliary clearance at the stenosis that causes accumulation of mucus at the undersurface of the stenosis and encroaches on the airway requiring coughing to blow this mucus past the stenosis in order to relieve this dynamic narrowing. It is important to realize that as the airway becomes more narrow, minor changes result in much greater symptoms (i.e., the change from 7 to 6 mm results in much greater symptoms compared to the change from 13 to 12 mm).
Etiologies
In the modern industrialized world, laryngotracheal stenosis is mainly an iatrogenic condition, the end process of airway damage from endotracheal intubation. It is estimated that anywhere from 0.9% to 8% of intubations lead to some form of identifiable long-term sequelae. Factors that are important to the development of laryngotracheal stenosis include prolonged intubation (as in the ICU setting for greater than 1 week), traumatic intubation with disruption of the laryngotracheal mucosa, intubation with too large a tube or with too small a tube where the balloon pressures required to maintain a seal cause mucosal ischemia, or movement of the tube due to swallowing or repeated neck flexion and extension. ICU patients are more susceptible in a multifactorial way with the interplay of the underlying medical condition, reduced ability to heal, and the bathing of the airway in a combination of gastric and oral secretions due to altered sensation and altered consciousness.
In the minority of patients, laryngotracheal stenosis is not the result of intubation injuries. Because there are conditions where we can intervene and halt the process of stenosis, it is important to mention them. Inflammatory or immune system problems such as Wegener’s granulomatosis, sarcoidosis, pemphigoid, and amyloidosis can present with airway narrowing, and each has a different management scheme that should be followed. Other causes include airway tumors, the sequelae from direct damage from surgical procedures meant to deal with other distinct problems, and tracheostomy tubes inadvertently placed through the cricothyroid space (intralaryngeal placement) instead of into the trachea.
There is a group of patients who have a presentation that is, as of yet, considered to be idiopathic. These patients are almost exclusively female, tend to present at a moderately early age (40s to 60s), and do not have a history of intubation within several years of the onset of symptoms. In some cases, there is no history of intubation. It is important to respect our ignorance in understanding the etiology of their stenosis and resist overly aggressive treatment that may leave them with severe postsurgical changes to the voice and persistent disease.
Laryngopharyngeal reflux has been identified as a cofactor in stimulating and promoting the formation of laryngotracheal stenosis. Stomach acid and enzymes (pepsin) make their way up the esophagus, and microaspiration events during silent reflux introduce small quantities of these caustics materials into the upper airway causing inflammation and an environment conducive to an exaggerated healing response after minor airway mucosal disruption. LPR alone cannot account for the episodes of idiopathic subglottic stenosis since men and women both are known to reflux but only women present with idiopathic subglottic stenosis.
Surgical Treatment
Treatment of laryngotracheal stenosis is highly dependent on the particulars of the etiology. Although the vast majority of cases are related to a clear-cut history of intubation injury, care must be taken to rule out the less common but potentially medically treatable and potentially fatal disorders such as Wegener’s granulomatosis. This chapter will focus on the surgical management of laryngotracheal stenosis.
Surgical treatments can be divided into endoscopic and open interventions, each possessing inherent advantages and disadvantages that we will discuss. It is important to realize that the choice of an endoscopic or open procedure should be made after synthesizing all of the available patient data and formulating a plan to treat the patient symptoms in partnership with the patient. The designation “endoscopic” or “open” defines an approach but tells us nothing about the actual procedures to be performed. Endoscopic approaches can be further subdivided into procedures that can be performed with the patient awake in the clinic setting or those performed in the operating room. With few exceptions, open procedures are performed in the operating room under general anesthesia (the major exception being tracheotomy that can be performed at the bedside under straight local anesthesia).
Endoscopic Approaches for Diagnosis and Treatment
Although the initial in-office examination typically includes an endoscopic examination using a flexible nasolaryngoscope that is used to assess laryngeal function and visualize the subglottic larynx and the trachea, the traditional endoscopic procedures are performed in the operating room under general anesthesia with highly variable techniques of ventilation due to institutional/surgeon/anesthesia personnel preference ranging from jet ventilation, endotracheal intubation, use of a laryngeal mask airway (LMA) to apneic techniques. Techniques that do not require intubation of the stenotic area such as jet ventilation or the apneic method do not disrupt the airway mucosa since a large tube is not introduced through the stenosis. This allows for an accurate assessment of the nature of the stenosis. Intubation many times alters the airway by introducing bleeding and dilating the stenosis as the tube pushes past. The largest direct laryngoscope that will fit into the laryngeal introitus should be used as the larger size offers more room to introduce and manipulate instruments. A video system with a 0-degree Hopkins rod will allow visualization of the airway and a way to accurately measure the length and location of the stenosis. The stenosis can be palpated to determine how fibrotic it is. All of this information should be recorded in a systematic fashion and will be used to formulate appropriate treatment options.
After a thorough visual investigation of the airway, biopsies can be sent to exclude other causes of stenosis including tumors, vasculitis, infection, and malignancy. At this time, even if the plan is to eventually perform a definitive procedure, the airway can be dilated to give the patient temporary relief from the effects of the stenosis. There are a variety of instruments to dilate the stenosis ranging from rigid to semirigid dilators that (Fig. 48.3) require passing progressively larger instruments into the stenotic segment to balloon dilators. Because balloons exert force in a radial manner, they exert their force directly on the stenosis, uniformly dilating without the associated collateral mucosal airway damage associated with introducing and removing multiple dilators. Balloons for airway dilation currently can be found in sizes ranging from 5 to about 20 mm (Fig. 48.4).




