26 Techniques and Devices for Lead Extraction
This chapter discusses the indications, techniques, and devices for lead extraction in detail, using definitions and information from the 2009 Heart Rhythm Society (HRS) Expert Consensus on Facilities, Training, Indications, and Patient Management for transvenous lead extraction.1
 Definitions
Definitions
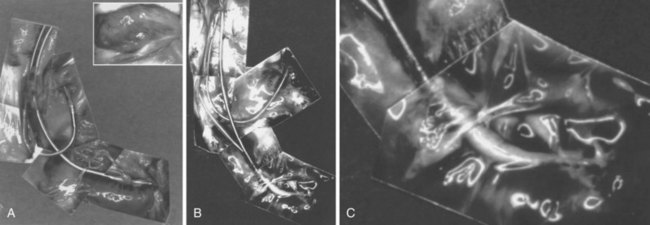
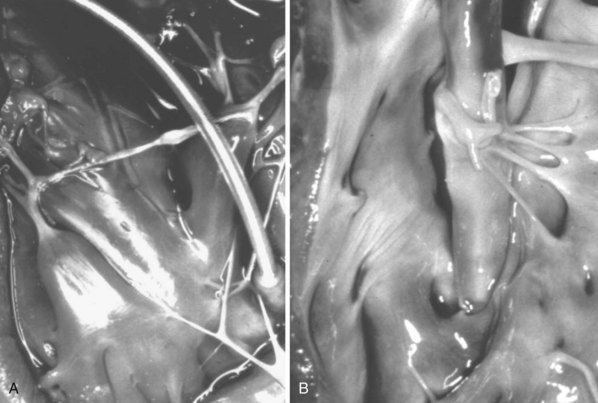
However, with prolongation of the duration of implantation, segments of chronically implanted leads are encased in encapsulating fibrous tissue and may be bound to the vein and/or heart wall, bound to another lead, or both (Figs. 26-1 to 26-4). Removal of chronically implanted leads from these binding sites becomes very difficult and potentially hazardous with traction alone. The tensile strength of encapsulating fibrous tissue is greater than that of the surrounding tissue, and thus leads cannot easily be removed without risking a tear or avulsion of the vein or heart wall. In such cases the removal, separation, and freeing of leads from encapsulating fibrous tissue are defined as lead extraction. As a rule, if one or more of the leads implanted in the patient that require removal are more than 1 year old or require extraction tools for removal, the procedure is labeled as a “lead extraction procedure,” and if not, then a “lead removal without extraction.”
Extraction techniques include traction, countertraction, counterpressure, and tissue disruption, cutting locally with an instrument, laser, or electrosurgery unit. Lead extraction techniques are designed to free the lead from the encapsulating fibrous tissue (countertraction) or to free the encapsulating fibrous tissue (counterpressure) from the vein or heart wall.2–4 Telescoping sheaths are used remotely to apply countertraction and counterpressure at the selected binding sites. Lead extraction procedures are those approaches used to apply the sheaths and remove the lead in a safe and efficacious manner.
Goals and Outcomes
The HRS consensus document, in an effort to unify nomenclature, suggested the following definitions1:
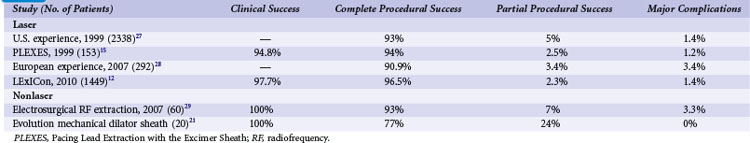
Although there are no published benchmarks, operators should strive for clinical success in 100% of patients with the least possible number of complications. Table 26-1 summarizes current rates of success and complications based on collective studies.
Intraprocedural complications are any complication that occurs during the procedure, as recorded from the time the patient enters the operating or procedure room to the time the patient leaves. This includes all preparation, from administering anesthesia to groin access to closing the incision and reversal of anesthesia. Postprocedural complications are any events that become evident within 30 days after the intraprocedural period. Major complications are any life-threatening complications, those that result in death, or any complication that results in persistent or significant disability or significant surgical intervention. A minor complication is any undesired event related to the procedure that requires medical intervention or “minor procedural intervention” and does not limit, persistently or significantly, the patient’s function or threaten life or cause death (Box 26-1).
Box 26-1
Classification of Implant/Lead Complications
Major
 Indications for Extraction
Indications for Extraction
Considerable divergence of opinion surrounds the indications for lead extraction. However, the 2009 HRS consensus document has unified opinions to a large extent.1 Indications are divided into three major categories: infection, venous access, and broken or nonfunctional leads. Also, emerging indications include implant patients who require magnetic resonance imaging (MRI) scanning for diagnosis (Box 26-2).
Box 26-2
Indications for Transvenous Lead Extraction*
Infection
Class I
Thrombosis or Venous Stenosis
Class I
Leads Affecting Patient: Functional or Nonfunctional Leads
Class I
Class IIa (nonfunctional leads only)
Class IIb
Class III
Magnetic Resonance Imaging–Related Indications
Class IIb
CIED, Cardiac implantable electronic device; ICD, implantable cardioverter-defibrillator.
Data from Heart Rhythm Society Expert Consensus on Facilities, Training, Indications, and Patient Management. Heart Rhythm 6:1085-1104, 2009.
Infection
Infection remains the most common indication for extraction. Complete extraction is indicated in cases of definite CIED infection when there is erosion, pocket infection, lead vegetations, or sepsis (see Chapter 25). Extraction is also recommended in patients with a device and endocarditis or occult gram-positive bacteremia. Extraction is also reasonable in patients with a device and persistent occult gram-negative bacteremia. Antibiotic therapy should be considered adjunctive therapy, and pocket debridement with local relocation is only palliative.
To prevent further serious and potentially lethal complications, all device components, including leads, must be removed to cure the infection. Also, the morbidity associated with a local pocket infection, the lethal sequelae of septicemia, and the potential risk of infected thrombus formation in the heart are well known. Because leaving an infected lead in the body is potentially lethal, the risk of the procedure is clearly less than the risk of lead extraction; that is, the risk of not extracting far exceeds the risk of extracting. The risk of Staphylococcus aureus device infection without extraction was supported by a series of 33 patients from the Duke Medical Center; 10 of 21 patients (47.6%) died without lead extraction, and 2 of 12 (16.7%) died despite lead extraction, and none from lead extraction.5 The safety and efficacy of complete lead extraction, with debridement and delayed reimplantation at a remote anatomic site, were demonstrated in 123 patients at the Cleveland Clinic with device infection. Despite infections with a wide range of bacterial organisms, mostly coagulase-negative staphylococci and S. aureus, extraction was associated with no major complications. Infection recurred only in those four patients who had incomplete extraction or reimplantation concurrent with the extraction.6
Noninfected Systems
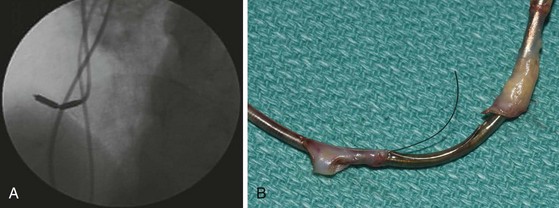
When functional or nonfunctional leads pose immediate risk, such as in a patient with life-threatening arrhythmias from a retained lead or fragment, or when the lead design poses a risk of perforation (e.g., Accufix with protrusion of J stylet outside lead), the decision to extract is straightforward (Fig. 26-5). Extraction of functional or nonfunctional leads becomes controversial when there is no immediate threat to the patient, because it is possible to abandon a failed or a nonrequired lead and implant ipsilaterally if the vein is patent, or contralaterally or even transiliac if the ipsilateral vein is occluded. The controversy persists because it is difficult to calculate the risk/benefit ratio of lead extraction versus abandoning such leads. Thus, when considering extraction of noninfected leads, it is important to balance the risk of extraction with the patient’s situation and to factor in the operator’s experience and not just literature data. Some operators may choose to extract only when there is infection; others who are more experienced may elect to extract all leads that are not required, whether functional or not, when the opportunity arises.
Leads Associated with Risk to Patient
In the case of functioning leads, the HRS document specifies that extraction of these leads may be considered when the lead design poses a potential risk in the future, as with the Accufix lead before J-wire fracture or protrusion. Abandoned functional leads that may pose interference risk or those no longer required can be considered for extraction, particularly in the absence of contraindications (see Box 26-2).
Thrombosis or Venous Stenosis
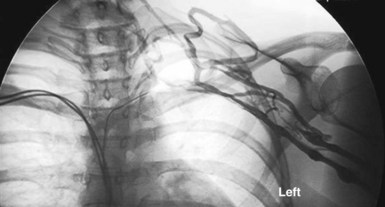
Lead extraction is indicated for patients with clinically significant thromboembolic events with evidence of clot on the lead, as well as in patients with superior vena cava (SVC) or subclavian vein stenosis or occlusion with symptoms. Lead extraction is also recommended in cases of bilateral occlusion for the creation of a conduit or for planned stent deployment (Fig. 26-6). When there is a contraindication to implantation on the contralateral side (e.g., arteriovenous fistula), extraction for creation of a conduit is recommended; otherwise, extraction is considered reasonable.
Creation of a Conduit
Sometimes, when there is severe stenosis with or without symptoms from the obstruction of flow, physicians have initiated balloon venoplasty and stenting without extraction of the leads. This is particularly difficult if either infection or reocclusion occurs, because extraction now becomes impossible without extensive open-heart surgery (Fig. 26-7). A more appropriate approach includes extraction, venoplasty, stenting, and reimplantation through the stent, as reported by Chan et al.7 in a subclavian occlusion that progressed to an SVC occlusion.
Inactive Leads: Functional and Nonfunctional but not in Use
A rationale for extraction of inactive leads is not as straightforward as for active leads. This situation differs from creation of a conduit. For example, if the ipsilateral vein is patent, insertion of a new left ventricular lead should be uneventful. However, the addition of a new ICD lead and abandonment of the original ventricular pacemaker lead creates an inactive, abandoned lead. The disposition of inactive leads is controversial, confusing, and at times emotional. Many questions need to be answered. Why should a lead be removed if it is not causing a problem (e.g., penetration, perforation, arrhythmia), is not dislodged, and is not broken?8 (See Box 26-2.)
 Risks and Outcomes
Risks and Outcomes
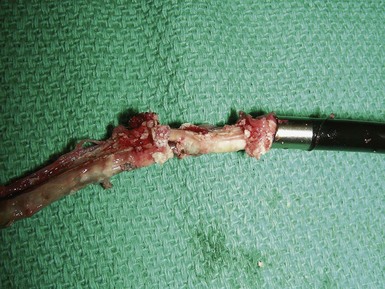
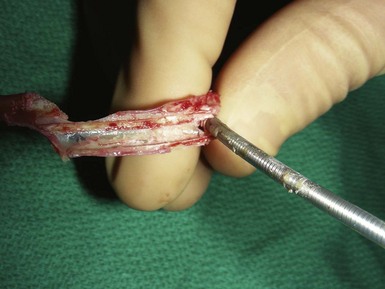
The risks associated with lead extraction are tamponade caused by intrapericardial vascular disruption of the SVC or heart; hemothorax from extrapericardial vascular tear outside the pericardial sac and into the thorax; arteriovenous fistula or dissecting hematoma (e.g., aortic arch); and failure to complete the lead extraction (Fig. 26-8). The latter is usually not considered a risk; however, a failed lead extraction may lead to additional procedures or may be a precursor for dangerous situations in the future.
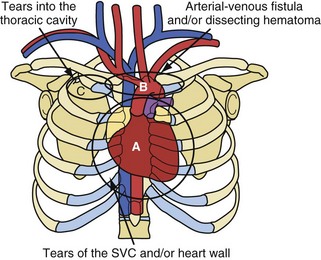
Figure 26-8 Potentially lethal complications from vascular tissue disruption during lead extraction.
Extraction centers from the continental United States and Hawaii voluntarily submitted data for a national registry between December 1988 and December 1999.9–11 The most recent published report, from 1996, included data from 226 centers, 2338 patients, and 3540 leads and demonstrated major complications in 1.4% of the cases (<1% for centers with >300 extraction procedures).10,11 The total U.S. data included 7823 extraction procedures and 12,833 leads (presented in 2000). Multivariate analysis of the data from 1994 through 1999 demonstrated four predictors of major complications (1.6%): (1) implant duration of oldest lead, (2) female gender, (3) ICD lead removal, and (4) use of laser extraction technique. Major complications were (1) death, 0.3%; (2) nonfatal hemopericardium or tamponade, 0.7%; (3) nonfatal hemothorax, 0.2%; (4) transfusion for bleeding/hypotension, 0.1%; (5) pneumothorax requiring a chest tube, 0.1%; and (6) other nonfatal events, 0.2% (including 4 arteriovenous fistulae, 2 pulmonary embolisms, 2 thoracotomies for defibrillator leads trapped in sheaths, 2 respiratory arrests, 2 strokes, 2 cases of renal failure, 1 anoxic encephalopathy, and 1 open-heart retrieval of a device fragment). In the more recent LExICon study of data from 13 centers in the United States and Canada, procedural success was 96.5%, with 97.7% clinical success.12 In LExICon, 1449 patients underwent laser-assisted lead extraction of 2405 leads. Failure to achieve clinical success was associated with body mass index (BMI) of 25 kg/m2 or less and low-extraction-volume centers. Procedural failure was higher in leads implanted for more than 10 years and when performed in low-volume centers. Major adverse events in 20 patients were directly related to the procedure (1.4%), including four deaths (0.28%). Major adverse effects were associated with patients with BMI less than 25 kg/m2. Overall, all-cause in-hospital mortality was 1.86%.
Potentially lethal complications requiring extensive surgical procedures include tear of the vein and heart wall causing tamponade, arterial tears causing arteriovenous fistula or dissecting hematoma; and tears into the thoracic cavity causing a hemothorax (see Fig. 26-8). The procedure-related complications are discussed in detail later. Time and surgeon experience are the two factors related to survival. Low blood pressure and poor tissue perfusion are time-dependent events. Being prepared for a cardiovascular emergency is the only way to meet time constraints. This includes having a cardiovascular surgeon immediately available, along with the proper instrumentation and experienced support personnel. A cardiovascular surgeon has the technical skill to manage these complications but may need direction from the extractor on the proper approach. Once a complication resulting in poor or no perfusion is recognized, the repair should begin immediately. The concern of needlessly subjecting the patient to extensive surgery and morbidity pales in comparison to that of applying the therapy late because of confusion or procrastination. Failure to recognize the complication in a timely fashion or the lack of access to qualified personnel may cause a lethal outcome.
 Clinical Considerations
Clinical Considerations
Patient Information and Preparation
The patient’s blood should be typed and crossmatched for a possible blood transfusion. A current chest radiograph and electrocardiogram (ECG) are mandatory (Fig. 26-9). An echocardiogram is mandatory for two groups of patients before the procedure, even if transesophageal echocardiography (TEE) is routinely available in the procedure room: those with infection, to rule out vegetation in the right atrium; and those with heart failure, to define cardiac function.