Fig. 16.1
Anatomy of the mesenteric vessels with rich collateral network (By permission of Mayo Foundation for Medical Education and Research. All rights reserved)
Arterial embolism has been reported as the most common mechanism accounting for 40–50 % of cases of AMI; it accounted for >50 % of cases treated at our institution over the last two decades [2, 3]. The acute takeoff of the SMA from the aorta makes it the most vulnerable vessel to embolization, most frequently an embolus arising upstream in the arterial circulation, typically the heart or the thoracic aorta. The majority of emboli originate from thrombi in the left atrium in the setting of atrial fibrillation, in the left ventricle secondary to hypokinesis/aneurysm following myocardial infarction or upon cardiac valvular lesions/synthetic valves. The typical patient may have an acute change in his/her cardiac status including an episode of atrial tachyarrhythmia, congestive heart failure, myocardial infarction, ventricular aneurysm, or failure to maintain a therapeutic anticoagulation status with a known chronic tachyarrhythmia [4, 5]. Aortic thromboembolism is a less frequent source of AMI, arising from thrombus atop ulcerative atherosclerotic plaques. It may occur spontaneously; however, the commonest predisposing factor is an endovascular procedure in the aorta (arteriography, intra-aortic balloon placement). It can also occur during open procedures with placement of a high aortic clamp on a diseased, shaggy aorta [6–11].
Diagnosis of Mesenteric Embolism
Patient Population
The hallmark of clinical presentation of a patient with AMI is abrupt onset, severe, nonlocalized “abdominal pain out of proportion to physical signs.” Identifying important clues and risk factors in the clinical history can often help make a timely diagnosis. These include: atrial fibrillation (AF), recent myocardial infarction, congestive heart failure, and prior embolic events. Not infrequently anticoagulation is interrupted in an elderly patient with chronic AF for an invasive procedure or due to fall risk [12]. In addition, a history of symptoms suggestive of chronic mesenteric ischemia (postprandial pain, weight loss, “food fear”), previous venous thromboembolic events, hypercoagulable states, and vasopressor therapy can point to a different etiology of the AMI.
Preoperative Testing
There is no laboratory study that confirms the presence of mesenteric ischemia. Furthermore, laboratory findings are often nonspecific and unaltered in the early stages, and delaying diagnostic imaging till results of laboratory tests are available in a suspected patient significantly decreases survival [13]. In the later stages, there is evidence of hemoconcentration from sequestration of fluid in the bowel wall including leukocytosis and elevated serum lactate and amylase. By the time these occur, there has usually already been compromise of bowel viability. Experimentally in a rat model, it has been suggested that the D-dimer level after two hours from insult may correlate with the presence of AMI [14]. Further, plasma and urine levels of intestinal fatty acid-binding protein (FABP) have been linked to bowel infarction and have been suggested as tools in the early diagnosis of AMI [15–17].
Preoperative Imaging
Computed tomography angiography (CTA) with intravenous contrast is now widely available and has replaced mesenteric arteriography as the definitive diagnostic tool in contemporary practice [18, 19]. It is a fast, effective, and noninvasive way to rule out commoner causes of acute abdomen, confirm the diagnosis of AMI, and potentially identify the etiology [20]. In addition, CTA differentiates between acute mesenteric thrombosis when an ostial occlusion of a calcified SMA is usually seen and acute arterial embolism when a more distal SMA occlusion of a noncalcified SMA is demonstrated. Typically emboli lodge at a branch point in the vascular tree, usually 1–2 cm distal to the origin of the vessel, most often at the takeoff of the middle colic artery branch, which results in the typical sparing of the proximal jejunum and distal transverse colon (Fig. 16.2). No one CTA finding is diagnostic of AMI; however, a combination of pneumatosis intestinalis, superior mesenteric artery occlusion, portomesenteric venous gas, or portomesenteric venous thrombosis together yields a positive predictive value of 100 % and a negative predictive value of 96 %, respectively. CTA is considered today the gold standard in diagnosis of AMI and should be the one and only imaging study ordered [19, 21].
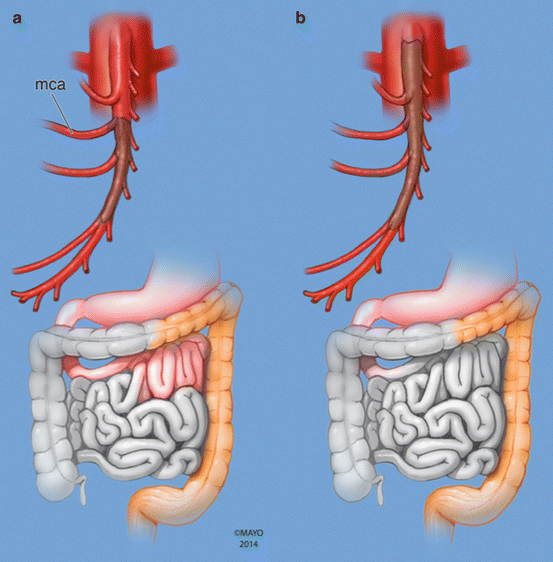

Fig. 16.2
Diagrammatic representation of typical location of embolic (a) and thrombotic (b) occlusion of the SMA with corresponding pattern of extent of bowel ischemia. (a) Embolic occlusion of the SMA at the branching point of the middle colic artery (mca). (b) In situ thrombosis of the SMA starting at the ostium (By permission of Mayo Foundation for Medical Education and Research. All rights reserved)
The role of mesenteric arteriography, long held as the “diagnostic gold standard” has evolved to therapeutic indications mostly in the treatment of in situ mesenteric arterial thrombosis with angioplasty with or without stenting [2, 22]. Additional applications include injection of intra-arterial vasodilators, thrombolysis, and aspiration thrombectomy [23, 24].
Additional noninvasive imaging modalities traditionally useful in the elective setting do not have a significant role to play in the diagnosis of AMI, where time is of the essence. Plain abdominal radiography is usually nondiagnostic; it only demonstrates signs of mesenteric ischemia when the process is very advanced, such as pneumatosis intestinalis and portal venous gas (Fig. 16.3) that herald a very poor prognosis in this setting [25].
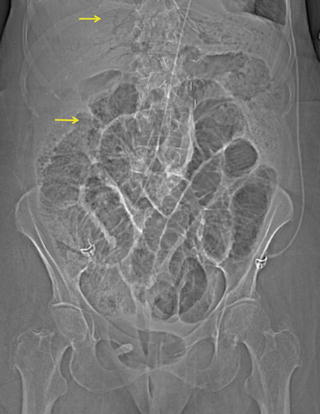

Fig. 16.3
Plain abdominal radiograph with evidence of portal venous gas and pneumatosis intestinalis (arrow)
Duplex ultrasonography is a very useful screening tool to identify stenosis of the mesenteric vessels in the elective setting [26, 27]. However, it is virtually useless in the acute setting due to poor visualization of the mesenteric vessels as a result of bowel gas from distention combined with significant patient discomfort. Similarly, magnetic resonance angiography (MRA) is theoretically appealing because of lack of the potential nephrotoxicity and allergic reaction associated with intravenous iodinated contrast for CTA; however, it is too uncomfortable and time consuming for a patient with significant abdominal pain to provide meaningful visualization of the mesenteric vasculature [28].
Preoperative Considerations
Prompt operative intervention is necessary for the treatment of acute mesenteric ischemia. Ideally, however, resuscitative efforts should be started as soon as the diagnosis is suspected and continued in the operating room. The initial management is threefold, including optimization of oxygen delivery with supplemental oxygen, intubation and mechanical ventilation, and transfusion of red blood cells to achieve a hematocrit of 30 %. Secondly, preservation of intestinal blood flow in the form of dynamic goal-directed fluid resuscitation, systemic heparinization titrated to a partial thromboplastin time of 60–80s and the use of selective vasoactive and inotropic agents to improve cardiac output. Lastly, initiation of treatment of sepsis by administering broad-spectrum antibiotics to treat bacterial translocation [29]. Further, at the time of induction of anesthesia, the patient should undergo adjunctive invasive monitoring including arterial line, central venous line, and Foley catheter placement [4].
Surgical Treatment
Once a diagnosis of AMI has been made, occlusion of the SMA has been confirmed, and the etiology of this occlusion determined, urgent bowel evaluation needs to be undertaken. All patients with suspicion of threatened bowel require prompt abdominal exploration. With the advent of minimally invasive techniques, one might suggest laparoscopy as an option. However, this has been only successfully demonstrated in a porcine model [30]. Sauerland et al. in 2006 clearly delineated that laparoscopy in the setting of an acute abdomen resulting from AMI offers very limited therapeutic benefit. Further, a limited view of the entire abdomen does not guarantee adequate recognition of ischemia [31]. Prior to midline laparotomy, the surgeon should be prepared for multiple modes of treatment of SMA occlusion, including: SMA thromboembolectomy, bypass, or retrograde stenting depending upon the final assessment. The patient should be prepped for possible great saphenous vein harvest (GSV) for an interposition graft/patch angioplasty. During laparotomy, the entire small and large bowel should be evaluated for signs of ischemia, by direct observation and peristalsis, assessment of pulses in the bowel mesentery by Doppler, and/or the use of fluorescein and ultraviolet light (Fig. 16.4) [32]. The latter technique has been reported to have a sensitivity of 96 % and a specificity of 95 % in the recognition of small bowel compromise [33]. It is recommended that revascularization be achieved prior to any bowel resection unless perforation causes significant spillage of enteric contents. Further, any necessitated small bowel resection should be limited to only necrotic segments. All segments with ischemic changes and equivocal viability should be preserved because once revascularization is achieved, these “questionable” bowel segments may recover. In the rare event that the entire small bowel demonstrates necrotic changes as a result of profound ischemia, neither further exploration nor revascularization should be undertaken as complete necrosis of the small bowel is not compatible with meaningful life. The abdomen should be closed and comfort care measures instituted.
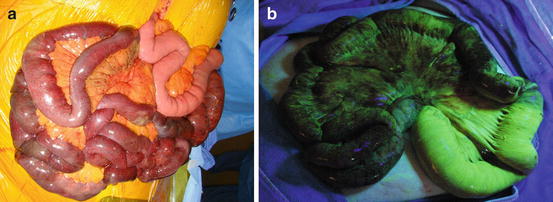

Fig. 16.4
Evaluation of small bowel during abdominal exploration. (a) Direct visualization and exploration facilitates the assessment of viability of the small bowel; note the sparing of the proximal jejunum. (b) Same patient as in (a). Assessment of the small bowel using fluorescein and ultraviolet light (Wood’s lamp)
Embolectomy of the Superior Mesenteric Artery
Once the bowel has been appropriately addressed, SMA revascularization is undertaken. The SMA can be exposed from an anterior and/or a lateral approach, both being equally effective. The anterior approach (Fig. 16.5a) ideally suited for embolectomy is performed through the base of the transverse mesocolon at the root of the mesentery without mobilization of the ligament of Treitz. To expose the main trunk of the SMA, the transverse colon is retracted cephalad and the small bowel caudad, which will allow for palpation of the vessel at the root of the mesentery, along the inferior margin of the pancreas especially if pulsatile or calcified. The peritoneum overlying it is next incised at the base of the transverse mesocolon, and careful dissection is carried down to the artery. It can often be difficult to expose due to the lack of pulsatility and significant mesenteric edema. It lies to the left of the superior mesenteric vein, and multiple small venous tributaries crossing the SMA may require ligation and division to facilitate exposure. The middle colic artery can also serve as a landmark to identify the SMA. It can be identified easily in the transverse mesocolon and traced proximally to its origin from the superior mesenteric artery. Careful dissection is necessary to not damage lymphatics, autonomic nerve fibers, the pancreas superiorly, and the delicate proximal jejunal branches coming off the SMA. If more proximal exposure of this vessel is necessary, this can be judiciously performed by carefully retracting the inferior pancreatic border cephalad. The lateral approach (Fig. 16.5b) to the SMA involves taking down the ligament of Treitz and mobilizing the entire small bowel to the right side of the abdomen. This provides access to an adequate length of the main trunk of the artery for direct revascularization without easy access for embolectomy of the distal branches. It is ideally suited for revascularization of the SMA with antegrade bypass from the supraceliac aorta or retrograde bypass from the infrarenal aorta or either iliac artery to treat in situ thrombosis.
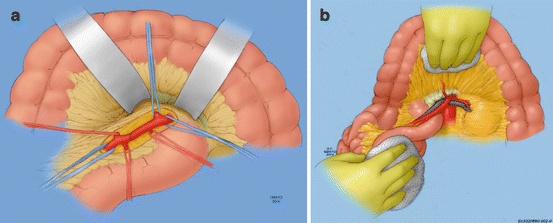

Fig. 16.5
Exposure of the superior mesenteric artery. (a) Anterior exposure of superior mesenteric artery. (b) Lateral exposure of superior mesenteric artery (By permission of Mayo Foundation for Medical Education and Research. All rights reserved)
Once the SMA and its branches have been isolated, therapeutic heparinization is confirmed, and proximal and distal control of this vessel is obtained. A transverse arteriotomy is performed in the infra-pancreatic segment of the artery. Rarely a longitudinal arteriotomy and closure with a patch are necessary, only if the artery is very small in caliber or diseased/stenotic. Following arteriotomy, the thrombus is extracted with 3–4 mm Fogarty balloons. Thromboembolectomy is performed by passage of the balloon catheter proximally as well as distally down the main trunk and into the branches if necessary, with special care not to force the catheter or overdistend the balloon and disrupt the delicate branches. Rupture of these branches that are relatively unsupported in the mesentery can result in an impressive hematoma, potentially further compromising bowel blood supply. This process is repeated until a clean pass of the balloon catheter is achieved (Fig. 16.6). After all thrombus has been removed, appropriate forward and backward bleeding followed by careful flushing with heparinized saline (10 units of heparin per mL) is performed. The arteriotomy is then closed primarily with interrupted 6–0 Prolene sutures, or with a GSV/bovine pericardium patch, and flow is restored (Fig. 16.7). At this point, reassessment of the bowel is recommended using a handheld Doppler, checking signals along the mesenteric and anti-mesenteric border of the bowel. Once SMA revascularization has been achieved and confirmed, obviously necrotic areas of bowel are resected but bowel continuity is not restored at this stage. Again, all equivocally viable bowel is preserved for reassessment at a later stage, and temporary abdominal closure is performed.
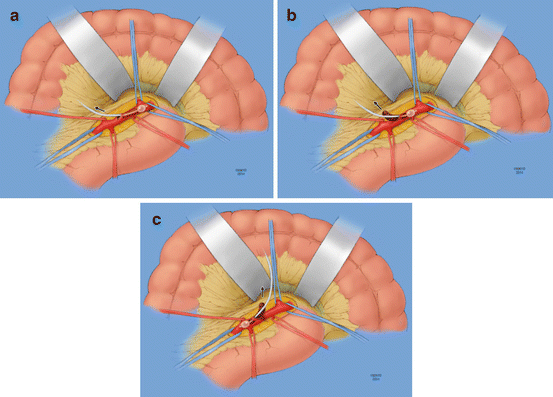

Fig. 16.6




(a–c) Balloon catheter embolectomy of the SMA, proximal and distal (By permission of Mayo Foundation for Medical Education and Research. All rights reserved)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


