Technique of Carotid Artery Stenting
Piotr Sobieszczyk
Charles Kerber performed the first balloon angioplasty to treat atherosclerotic carotid artery stenosis in 1980. Since then, the intervention evolved from “plain old” balloon angioplasty to a procedure which utilizes finely engineered embolic protection devices and nitinol self-expanding stents. While the experience from large-scale clinical registries and randomized controlled trials may not have settled the debates between proponents of surgical endarterectomy (CEA) and carotid artery stenting (CAS), endovascular therapy has been established as a durable and safe treatment for extracranial carotid artery disease. The inherent advantage of CAS relies on its minimally invasive, percutaneous approach. It is particularly attractive for patients who, for clinical or anatomical reasons, are at high risk of complications when undergoing surgical revascularization (Table 28.1). The Federal Drug Administration approved CAS for such high surgical risk patients in 2004 and in 2011, and are considering expanding the indications to include patients with normal surgical risk. Some societal guidelines support its use in selected patients with symptomatic and asymptomatic carotid artery disease.
As the clinical experience with CAS grew, it became clear that some patients are also at high risk of procedural complications with CAS. As with any invasive therapy, procedural safety and success depend heavily on appropriate patient selection.
The clinical indications for CAS are identical to those used for selecting patients for surgical revascularization. Patients with symptomatic carotid artery stenosis exceeding 50% and those with asymptomatic lesions greater than 70% may be considered for CAS although not yet approved by CMS. It is the preferred treatment in patients who have neck anatomy and comorbidities unfavorable for CEA. The threshold for any revascularization in asymptomatic patients with moderate to severe disease has increased with the advent of more effective medical therapy. Revascularization is still considered reasonable in some patients with carotid stenosis exceeding 70% provided their perioperative and periprocedural risk of death, stroke, and myocardial infarction (MI) is low. CAS may be considered in patients with difficult neck anatomy and medical comorbidities which may increase the risk of perioperative complications.
Table 28.1 High Surgical Risk Criteria. Adopted from SAPPHIRE trial, CAPTURE Registry | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The experience emerging from randomized clinical trials and large registries conducted in the last decade, supports the notion that some patients have a higher risk of complications when undergoing endovascular therapy for carotid artery stenosis. These high-risk features may be anatomical or may be related to patient’s clinical characteristics. While none have been formally established, clinical experience identified a number of these high-risk features: Age over 80 years, complex aortic arch anatomy, ulcerated and thrombotic plaque.
Any deviation from normal arterial anatomy will increase the technical difficulty of the procedure and challenge the operator. Whether the challenge comes at the access site, embolic protection device landing zone in the distal internal carotid artery or anywhere in-between, it will inherently increase the risk of procedural complications. Thus, every stage of the procedure must be examined when evaluating patient’s candidacy for CAS. CAS may be contraindicated in patients with advanced peripheral artery disease and no femoral arterial access. While the procedure may be performed via radial or brachial artery, lack of familiarity with such access introduces another degree of difficulty and risk.
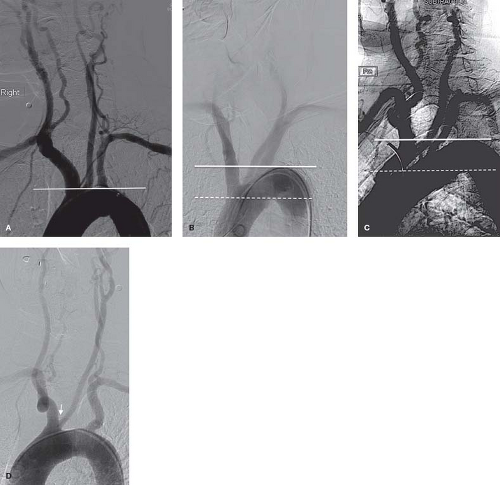
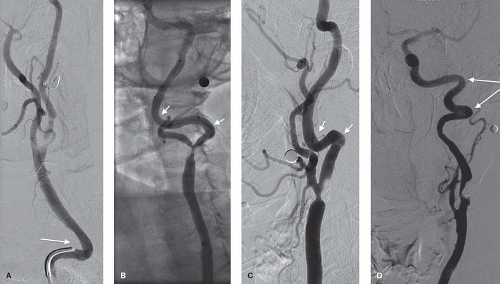
A hostile aortic arch anatomy introduces geometrical challenges, which increase the complexity of catheter manipulations and increase the risk of embolic complications (Fig. 28.1). Complex anatomy and atherosclerotic disease of the aortic arch is associated with advanced age and is one of the reasons that octogenarians may have a higher risk of periprocedural complications. Excessive tortuosity of the common and internal carotid arteries will pose a challenge with delivery of the sheath, embolic protection device and stent (Fig. 28.2). Aggressive manipulations to accomplish these tasks will increase the risk of vessel dissection and embolization. The likelihood that these anatomical challenges will increase procedural complications is inversely related to the operator’s experience and skill. Lesion-specific features should also be considered when considering candidacy for an endovascular intervention. A densely calcified lesion may be resistant to balloon dilation and hinder stent expansion. A thrombotic lesion carries a higher risk of distal embolization. These lesions may be more suitable for open surgical approach.
In addition to high-risk anatomical features, there are clinical characteristics, which may render patients unsuitable for CAS. These may include severe contrast allergy, intolerance of dual antiplatelet therapy, or significant chronic kidney disease.
Another group deserving special consideration consists of patients who may be particularly intolerant of severe baroreflex response associated with CAS. The stent-induced mechanical stretch of the artery and carotid body initiates a physiologic
response, which causes hypotension and bradycardia of variable severity. These self-limited hemodynamic disturbances are usually well tolerated in patients with complex cardiovascular disease and do not require any intervention beyond administration of intraprocedural adenosine. In some patients, such as those with significant aortic stenosis, additional measures to support heart rate and blood pressure may be needed. The baroreflex response is uncommon in patients who previously underwent surgical endarterectomy.
response, which causes hypotension and bradycardia of variable severity. These self-limited hemodynamic disturbances are usually well tolerated in patients with complex cardiovascular disease and do not require any intervention beyond administration of intraprocedural adenosine. In some patients, such as those with significant aortic stenosis, additional measures to support heart rate and blood pressure may be needed. The baroreflex response is uncommon in patients who previously underwent surgical endarterectomy.
In general, CAS has been studies and developed for patients with atherosclerotic carotid artery disease. The technique has been used in some patients with carotid artery dissection, carotid artery vasculitis, aneurysmal disease, or trauma. The experience in these patients is limited, success is variable, and decision to consider CAS should be made cautiously.
Clinical Evaluation
With rare exceptions, most carotid artery interventions are elective and should be performed as stand-alone procedures rather than in combination with other invasive procedures. The elective nature of these procedures allows for a comprehensive preprocedural evaluation, which must include a detailed history taking, physical examination, and adequate imaging. The purpose of such evaluation is to define whether the patient is symptomatic or asymptomatic; to establish the neurologic baseline; to describe the severity and characteristics of the stenotic lesion; and define the periprocedural risk and suitability for carotid stenting, carotid endarterectomy, or medical therapy.
Clinical evaluation must elucidate whether symptomatic patients are truly symptomatic and confirm that asymptomatic patients are indeed asymptomatic. Other more likely causes of neurologic symptoms, such as atrial fibrillation, cardiac pathology, or aortic arch disease must be considered and excluded.
Carotid artery duplex ultrasound is the most commonly available preprocedural imaging modality. It provides information about the tortuosity of the cervical carotid segment plaque morphology and the degree of arterial calcification. In most cases it provides sufficient information to plan the procedure. Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) of the neck and brain may be valuable in patients with advanced age and those who are symptomatic to define both the anatomical challenges and baseline status of the involved hemisphere.
Preprocedural Pharmacotherapy
Antiplatelet therapy reduces the risk of stent thrombosis and periprocedural embolic events and must be initiated prior to the procedure. All patients should be treated with a daily dose of aspirin 81 to 325 mg. Clopidogrel at a daily dose of 75 mg should be started at least 5 days prior to procedure to achieve adequate platelet inhibition. Alternatively, a loading dose of 300 mg or, preferably, 600 mg is commonly administered on the morning of the procedure. The dosing strategy for clopidogrel is extrapolated from the cumulative experience derived from coronary interventions. The newer antiplatelet agents, prasugrel and ticargalore, have not been studied in patients undergoing CAS.
A more individual approach is needed for patients who receive chronic antithrombotic therapy. The risk of thrombotic complications will dictate whether these agents can be interrupted around the time of the intervention or whether transition to intravenous anticoagulant is warranted. With few exceptions, antithrombotic agents can be interrupted without a “bridging” therapy. While dual antiplatelet therapy with aspirin and clopidogrel is still desirable in patients on chronic antithrombotic therapy, the increased bleeding risk associated with these three agents may justify a single antiplatelet agent to be used after the procedure.
Because of the expected decrease in heart rate and blood pressure, antihypertensive agents and beta blockers should be withheld on the morning of the procedure. In patients with difficult to control hypertension requiring multiple agents, selective discontinuation of several agents may be appropriate to avoid excessive periprocedural hypertension. This precaution may not be necessary in patients treated for restenosis of prior CEA or stenosis proximal or distal to the carotid body. A thorough understanding of the baseline blood pressures and medical regiment will facilitate postprocedural blood pressure control and reduce the risk of hyperperfusion syndrome. Diuretic therapy may also be withheld in patients at high risk of contrast-induced nephropathy.
Patients with contrast allergy should be pretreated with prednisone, antihistamine and occasionally the addition of H2-pump inhibitor. Prednisone (40 to 60 mg) should be administered on the evening before and morning of the procedure. Alternatively, an intravenous dose of solumedrol (100 mg) can be given just before the procedure.
Patient Positioning and Arterial Access
The common femoral artery is easily accessible with the patient in the supine position and is the preferred access site. The patient’s head should be placed in a cradle to minimize head movement during diagnostic angiography and subsequent intervention. To optimize quality of the digital subtraction angiograms (DSAs), patients will be required to suspend respiration, stop swallowing and blinking—these instructions should be rehearsed before the procedure. In addition, patients should be warned that they might notice a metallic taste during cerebral angiography. CAS procedures are normally performed without or with minimal sedation to facilitate patient’s participation during diagnostic angiography and to allow for neurologic monitoring. Neurologic function can be measured by asking patient to squeeze a toy in the contralateral hand or by asking occasional questions. Nevertheless a small dose of short-acting benzodiazepine (midazolam) and opioid analgesic (fentanyl) are commonly administered and titrated to reduce anxiety and discomfort without hindering patient’s cooperation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree