Potential uses of biomarkers in asthma
Biomarker thresholds used for the selection of targeted asthma therapies (a) and effect of targeted therapies on biomarkers and clinical outcomes (b)
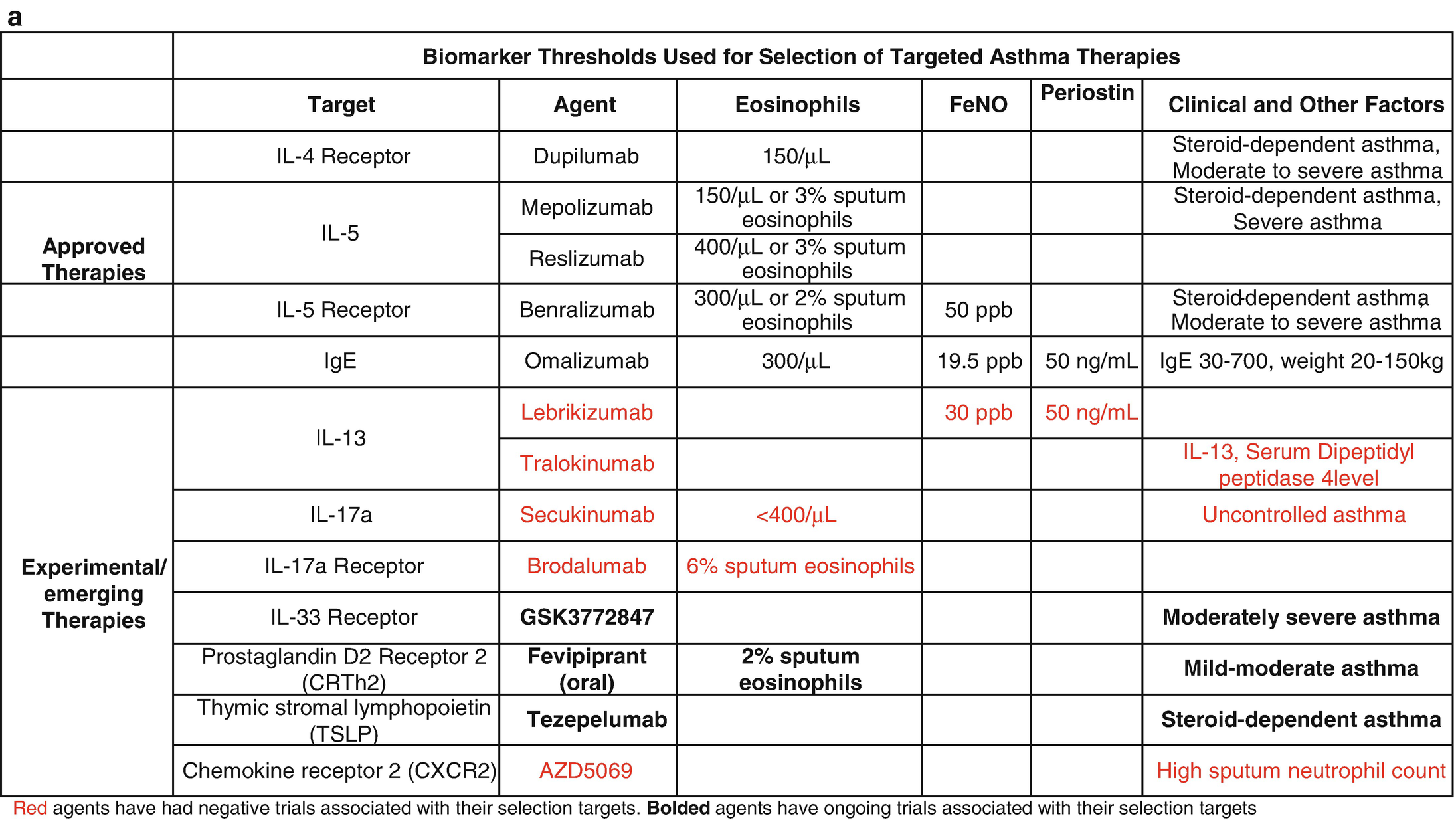
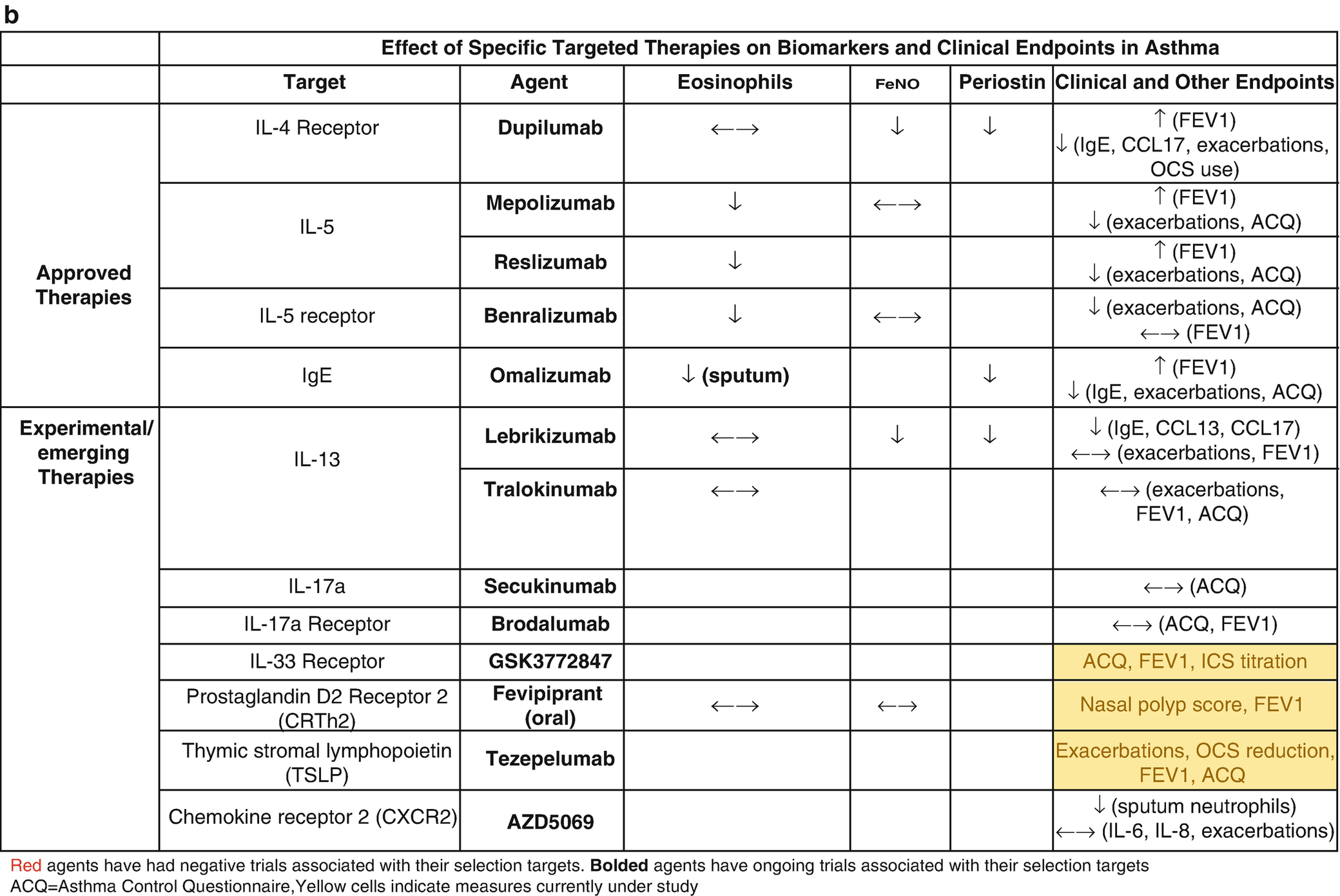
Efforts to identify new biomarkers have been fraught with a lack of prospective randomized controlled trial (RCT) data and the relatively slow pace of science. The most promising of these biomarkers was periostin, which was developed to identify patients with high type 2 (T2) inflammatory responses that would be more likely to respond to the anti-interleukin (IL)-13 biologic therapy, lebrikizumab. However, the phase III RCT trials, Lavolta I and II, that stratified patients by serum periostin levels failed to consistently reach their primary end points, so periostin remains unproven [2]. FeNO and DDP4 are also associated with high T2 responses but have not been prospectively studied in therapeutic trials [3]. Clinical features have also been used to subtype asthma using computational clustering algorithms and have shown some consistent phenotypes across multiple cohorts and methodology; however, they are not longitudinally stable and their utility in clinical practice has not been shown prospectively [4, 5]. Cross-sectional studies have identified transcriptomic signatures in the sputum, blood, nasal brush, and bronchial epithelium that are associated with clinical features suggesting that such subgrouping is possible, but again the longitudinal stability of these signatures remains in question and prospective pharmacogenomic studies remain to be reported. Here we will review the current biology and clinical use on the currently used biomarkers focusing on the practical aspects of their interpretation [6, 7]. It is important to note that, while promising, biomarkers in asthma are yet to meet their full prop osed application in clinical practice. At this point, their main utility remains in patient selection for biologic therapy (Table 8.1a).
8.2 Biomarkers Associated with Type 2 Inflammation in Asthma
T2 inflammation is described as inflammation mediated by upregulation of T2 inflammatory mechanisms. The term T2 was coined to encompass both T helper cell and type 2 innate lymphoid cell (ILC2) mediated inflammation as both cell types result in increased expression of type 2 cytokines including IL-4, IL-5, and IL-13. IL-5 results in increased downstream migration, survival, and production of eosinophils, IL-4 results in changes in eotaxin 3 levels that mediates homing of eosinophils and production of specific IgE which is critical to the allergic response, and IL-13 controls mucous hypersecretion and airway hyperresponsiveness [8]. Surrogate markers of T2 inflammation have been identified and will be covered in this section of the chapter.
8.3 Eosinophils
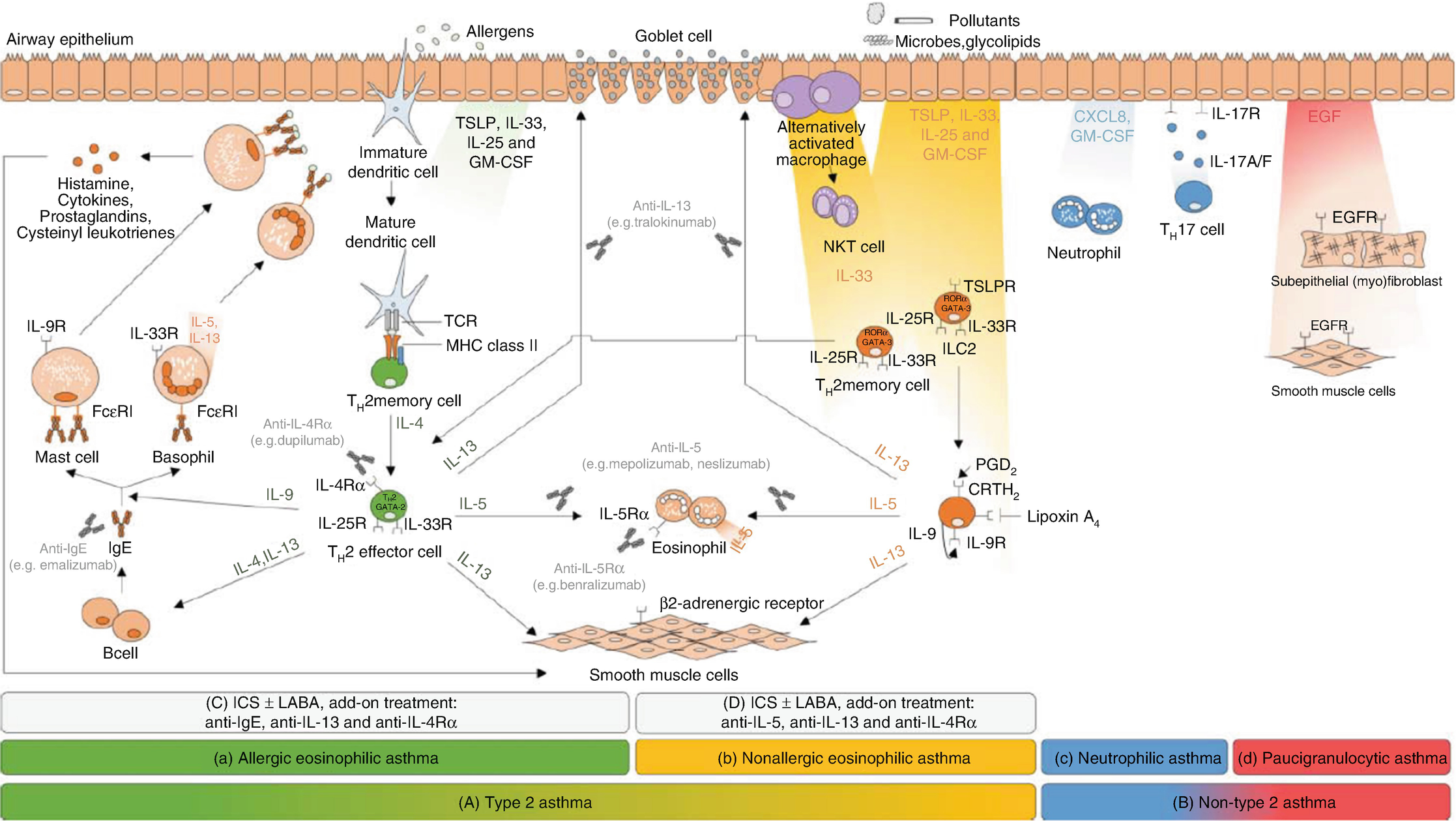
Schematic of four sputum inflammatory types and type 2 and non-type 2 inflammation. (Obtained from Godar et al. mAbs 10(1):34–45)
The importance of accurately identifying eosinophilic inflammation was highlighted by the initial studies of an anti-interleukin 5 biologic that proved ineffective when applied to the general asthma population [17–19] but proved highly efficacious when studied in patients with eosinophilic asthma [20, 21]. Following the approval of IL-5-targeted therapies, the use of eosinophilic biomarkers for patient selection and phenotyping has become standard in difficult-to-manage asthma (Table 8.1a). Eosinophilic airway inflammation can be assessed by invasive measures (bronchoalveolar lavage or endobronchial biopsies), noninvasive measures (sputum eosinophils, exhaled nitric oxide levels), or surrogates (such as peripheral blood eosinophil counts). Sputum and peripheral eosinophils are the most commonly used biomarkers. There is some controversy regarding the correlation of peripheral eosinophils with the gold standard sputum eosinophils. While early studies suggested that sputum eosinophils were more accurate markers of airway inflammation than peripheral eosinophils [22], a more recent study suggests that peripheral eosinophils function as reliably as sputum eosinophils in predicting asthma exacerbations [23]. A validation study in mild-moderate asthma demonstrated a statistically significant correlation coefficient of 0.59 between blood and sputum eosinophils, and a peripheral eosinophil cutoff of 270 cells/μL had sensitivity and specificity for eosinophilic inflammation of 78% and 91%, respectively [24].
Despite the limitations of blood eosinophils, they are quickly gaining favor as the preferred marker for eosinophilic inflammation because of the complexities and practical limitations associated with obtaining sputum markers. Sputum induction for eosinophils is not only cumbersome to perform but has a success rate of about 77% in terms of collecting airway cells and epithelial lining fluid [25]. There are also ongoing disagreements about the appropriate cutoffs for sputum eosinophils, but generally, counts ≥3% are considered significant. Currently, sputum-based biomarkers are only available at select asthma centers, and sputum largely remains a research tool. The threshold for peripheral eosinophilia varies based on its indications. Patients with serum eosinophils >250 cells/μL had lower FEV1 and worse asthma control than those with normal eosinophils counts [26]. For anti-IL5 therapy, the cutoffs vary based on the pre-determined values used for the primary analysis of the phase III trials for each drug: ≥150 cells/μL at the time of testing or ≥300 cells/μL in the previous year for mepolizumab, ≥400 cells/μL for reslizumab, ≥300 cells/μL or 150 cells/μl in patients on maintenance systemic steroids for benralizumab, and >150 cells/μL for dupilumab with no threshold for oral corticosteroid (OCS)-dependent asthma. There is significant variability in peripheral blood eosinophil counts, so caution should be used in interpreting a single value. The exact cutoff for patients on CS maintenance therapy is still not known as the impact of ICS and OCS on peripheral eosinophil counts is variable. The general consensus is that persistence of eosinophils despite maximal standard therapies and confirmed adherence indicates the likelihood of a positive response to currently available biologic therapies targeted at eosinophilic or T2 inflammation.
8.4 Immunoglobulin E (IgE)
IgE is an important marker of atopy and is the only blood-based biomarker available to identify allergic asthma. Serum IgE gene expression (as with blood and airway eosinophilia) is highly upregulated in T2 inflammation [27]. Total serum IgE level positively correlates with asthma severity in adults and in children [28]. In children, IgE levels also correlate with response to ICS, probability of wheezing and reduction in forced expiratory volume in 1 second (FEV1) [29, 30]. Multi-allergen IgE was the only biomarker considered as a core biomarker for atopic asthma by the National Institutes of Health (NIH) expert group for asthma biomarkers [31].
Omalizumab is a monoclonal antibody that targets free IgE and has been shown to be effective in a wide range of serum IgE levels. Although useful in phenotyping and selection of targeted therapy, total IgE level does not predict response to omalizumab (Table 8.1b). Instead, elevated eosinophil levels, high baseline ICS dose, and low baseline FEV1 were associated with a response to omalizumab therapy [32]. Therefore, the focus of clinical evaluation of patients with allergic asthma is on the identification of IgE specific for aeroallergens by immunoassay testing or skin prick testing (SPT) , which further aids in guiding allergen avoidance strategies. In summary, utility of serum IgE level is prim arily in identifying patients with allergic asthma phenotype.
8.5 Periostin
Periostin is an ubiquitous regulatory extracellular matrix protein involved in a variety of homeostatic and disease processes, which plays a crucial role in pathological lung remodeling. Periostin is upregulated by T2 cytokines, IL-4 and IL-13, and is believed to contribute to subepithelial airway fibrosis in asthma, though it has also been associated with idiopathic pulmonary fibrosis [33]. Its net function in asthma is unclear, as mouse models have demonstrated conflicting evidence of both protective [34] and pathogenic [35] roles in airway inflammation and hyperreactivity. Periostin is believed to augment eosinophil chemotaxis to inflamed airways by strengthening adhesion. It has been shown to be associated with increased levels of IL-4 and IL-13 and persistent airway eosinophilic inflammation in severe asthma [36]. Its utility as a T2 biomarker in asthma stems from this association with eosinophilic airway inflammation, which has long been associated with more severe asthma and airway remodeling [37].
Periostin has been utilized as a surrogate measure of T2 asthma with demonstrated efficacy in predicting responsiveness to therapy in severe asthma. Subjects in a phase II study of anti-IL13 antibody, lebrikizumab, for severe steroid-dependent asthma were stratified by periostin levels and demonstrated significant improvement in FEV1 in the “high periostin” group and none in the “low periostin” group [38]. A study of omalizumab demonstrated that increased levels of periostin pre-treatment were associated with a greater improvement in exacerbation frequency post-treatment [39]. As a result of these studies, it has been proposed that periostin be used when determining the next step in therapy, with the high periostin group qualifying for further consideration of agents targeting type 2 inflammation [40]. This strategy has not been validated in any studies however and currently is not the standard of care.
8.6 Fraction of Exhaled Nitric Oxide (FeNO)
The presence of endogenous NO was first described in 1991 [41] and since then has been studied extensively in asthma [42, 43]. FeNO production is associated with both underlying IL-13, atopic, and eosinophilic airway inflammation [44]. FeNO is both a diagnostic and predictive marker (Table 8.1a), and the presence of high FeNO denotes a T2 phenotype and is correlated with sputum eosinophils >3% [24, 45, 46]. The threshold that is used to ascribe T2 status is a subject of ongoing debate, and cut points of >20–25 parts per billion (ppb) are associated with T2 inflammation [43]. FeNO is a predictive marker of response to ICS, omalizumab, and dupilumab, with levels >50 ppb in adults and >35 ppb in children predicting a response to ICS [43]. Dupilumab rapidly decreases FeNO levels within weeks of initiation of therapy (Table 8.1b) [47]. The measurement of FeNO is now easily performed in a clinical setting using a portable analyzer (Niox Vero), and cost analysis studies have demonstrated that FeNO reduces costs by improving asthma control, decreasing exacerbations, and reducing cost of high- vs medium-dose ICS [48]. FeNO use has not been widely adopted except in sub-specialty clinics that manage asthma, despite its predictive and diagnostic capabilities. The use of FeNO in the management of asthma will be included as a topic for updated National Heart Lung Blood Institute (NHLBI) National Asthma Education and Prevention Program (NAEPP) guidelines.
8.7 Biomarkers Associated with Non-type 2 Inflammation
While T2 asthma has several clinically available biomarkers, non-T2 asthma remains a challenging disease to diagnose despite its estimated prevalence of approximately 50% in patients with mild to moderate asthma and a significant minority of severe asthma [27]. Non-T2 asthma is defined primarily by the absence of T2 features rather than the presence of a specific pathological finding and characterized by an attenuated response to glucocorticoids [49]. The lack of data demonstrating the activation of a unifying non-eosinophilic inflammatory pathway has limited biomarker identification and increased the belief that non-T2 asthma may instead represent a cluster of different mechanisms that have not been adequately elucidated.
Sputum inflammometry in patients with non-T2 asthma reveals a neutrophil-predominant or paucigranulocytic pattern [9]. Neutrophilic asthma is uncommon but associated with glucocorticoid resistance, poor outcomes, and higher healthcare costs [50]. Neutrophil-driven inflammation has been proposed as a source of non-T2 asthma development from a variety of environmental triggers that cause differing immune responses [51]. Based on this evidence, randomized controlled therapeutic trials targeting the neutrophil chemoattractant, C-X-C motif chemokine receptor 2 (CXCR2), were conducted but did not show a positive clinical effect (Table 8.1a, b). This has called into question the role neutrophils have in non-T2 asthma pathogenesis and raised suspicion that they may be an end-product or collateral marker of non-T2 inflammation rather than a key effector cell [52].
Despite disappointing outcomes from therapies that target neutrophils, the hope remains that neutrophil-driven inflammation and/or its associated cytokines may be able to predict asthma severity or type. IL-8 is a pro-inflammatory chemokine produced by macrophages and airway epithelial cells and is elevated in asthma patients and is an important ligand for CXCR2 function [53]. A recent study on peripheral blood mononuclear cell protein expression found that IL-8 performed poorly as a discriminatory biomarker in general but did show promise in its ability to distinguish severe from mild-moderate non-T2 asthma [54]. IL-17, an upstream cytokine used in the recruitment of various inflammatory cells including neutrophils, has been implicated in severe asthma in a variety of studies with an unclear pathological role [55, 56]. This is best demonstrated by the outcome of a phase II trial of the IL-17 inhibitor, secukinumab, that was terminated early by the sponsor due to lack of efficacy (Table 8.1a).
IL-6 has received special attention as a potential biomarker given its general pro-inflammatory effects and positive association with obesity and active viral infections, both of which are associated with severe asthma [57, 58]. IL-6 has also been shown to correlate with asthma severity [59], is measurable in sputum and blood in humans [59], and is known to facilitate non-eosinophilic mucous hypersecretion in mouse models of asthma [60]. Furthermore, blockade of the IL-6 pathway reduces airway hyperresponsiveness [61]. Despite these desirable characteristics, its utility as a biomarker is limited by its lack of specificity as it is also associated with chronic obstructive pulmonary disease and aging [62]. To date there have been no human studies targeting IL-6 in asthma, though animal studies have shown promise [60, 61], and the anti-IL-6R antibodies tocilizumab and sarilumab are both approved for use in rheumatoid arthritis [63] raising hope that a similar disease-modifying outcome could be achieved in asthma.
In summary, a vast body of data exists supporting the presence of neutrophilic inflammation, but its role in asthma pathophysiology remains controversial making the presence of high neutrophils an unreliable marker of non-T2 asthma. A recurring theme is that non-T2 asthma does not follow a single dominant pathway but rather represents a variety of mechanisms leading to different degrees of asthma severity. There are likely to be multiple non-T2 endotypes, each driven by a distinct mechanism. Therapeutic interventions targeting non-T2 asthma have been largely unsuccessful thus far, possibly due to a lack of discriminatory capability to identify subjects who will respond to these non-T2 interventions. Better biomarkers are desperately needed if we are to identify personalized therapies for patients with non-T2 asthma. Ongoing research focused on surrogate measures of the neutrophil-inflammatory cascade and other novel pathways driven by alarmins such as IL-33 and thymic stromal lymphopoietin (TSLP) are desperately needed. Otherwise, the definition of non-T2 asthma will continue to rely on the absence of markers of T2 inflammation, an unsatisfactory solution in an age where targeted therapy is desired and endotypes are sought.
8.7.1 Role of Biologics in Asthma
The successful use of monoclonal antibodies dates back to 1988 when Orthoclone OKT3 (an anti-CD3 antibody) was used to treat transplant rejection [64]. Since then these monoclonal antibodies have revolutionized care in rheumatology, oncology, toxicology, transplant medicine, and more recently, pulmonology. In asthma, the use of these biologics takes us one step closer to delivering precision medicine for a disease that is very prevalent and heterogeneous. It is important, however, to remember that experience with biologics is still evolving. There are still gaps in our ability to accurately correlate asthma phenotypes with endotypes, and available biomarkers guiding patient section for these drugs are not always accurate.
- 1.
Omalizumab (Xolair ™; Genetech, San Francisco CA and Novartis, Basel, Switzerland)
- 2.
Mepolizumab (Nucala ™; GlaxoSmithKline, Brentford, UK)
- 3.
Reslizumab (Cinqair ™; Teva Pharmaceutical, Petah Tikva, Israel)
- 4.
Benralizumab (Fasenra ™; AstraZeneca, Cambridge, UK)
- 5.
Dupilumab (Dupixent ™; Regeneron Pharmaceuticals, Tarrytown, NY and Sanofi Genzyme, Paris, France)
Biologics in asthma
Biologic agent | MOA | Patient selection | Dosing | Efficacy (Key outcomes improved) | Safety | Notes |
|---|---|---|---|---|---|---|
Omalizumab (Xolair ™) FDA approved in 2003 | Humanized IgG1 antibody that binds to the Cε3 domain of free IgE and prevents it from binding to FcεR1 | Age ≥6 years in the USA (≥12 years in the UK) IgE: 30–700 IU/mL Allergic sensitization | SC (dose of 0.016 mg/kg per IU of IgE) every 2–4 weeks based on age, weight, and IgE levels | AHR FEV1 Asthma symptoms PEFR OCS/ICS doses Rescue medication Exacerbations Hospitalizations | Anaphylaxis in 0.2% Injection site reaction 45% | Highest efficacy in Th2 high patients Potential disease-modifying drug |
Mepolizumab (Nucala ™) FDA approved in 2015 | Humanized IgG1 antibody that inhibits IL-5 from binding to the α-subunit of the IL-5 receptor complex expressed on eosinophils | ≥12 years Blood AEC of ≥150 cells/μL at the time of testing or ≥300 cells/μL in the previous year ≥300 cells/μL only in the UK | SC 100 mg every 4 weeks | FEV1 OCS dose Exacerbations Asthma symptoms QOL | 3% rate of adverse reactions (mostly mild) Warning against herpes zoster | Only moderate efficacy in patients with persistent sputum eosinophils |
Reslizumab (Cinqair ™) FDA approved in 2016 | Humanized IgG4 antibody that inhibits IL-5 from binding to the α-subunit of the IL-5 receptor complex expressed on eosinophils | Age ≥18 years Blood AEC ≥400 cells/μL in the previous year | IV infusion 3 mg/kg every 4 weeks | FEV1 Asthma control Exacerbation QOL | Anaphylaxis (0.3%) Higher rate of malignancy vs placed (0.6 vs 0.3%) Transient CPK elevation | Response is better with higher eosinophil counts |
Benralizumab (Fasenra ™) FDA approved 2017 | Humanized afucosylated recombinant IgG1 antibody that binds with high affinity to the α-subunit of the IL-5 receptor | Age ≥12 years Blood AEC ≥300 cells/μL in the previous year | SC 30 mg every 4 weeks for the first three doses then every 8 weeks | FEV1 Asthma symptom score Exacerbation OCS doses | Headache and nasopharyngitis Allergic reaction at higher rate than other anti-IL5 | Available for pre-filled auto injector (potential for home-based administration) |
Dupilumab (Dupixent ™) FDA approved in 2018 | Fully human monoclonal antibody to the alpha unit of IL-4 receptor. Blocks IL-4 and IL-13 | Age ≥12 years Moderate-to-severe asthma Blood AEC ≥300 cells/μL in the previous year | SC 200 mg or 300 mg every 2 weeks after initial loading dose | Exacerbations FEV1 OCS doses | Transient eosinophilia (over 3000 cells/mL) in 1.2% | Home administration is an option Anti-drug antibody in 2–5% patients |
Tezepelumab (received “breakthrough drug” designation in 2018. Not yet FDA approved) | Fully human monoclonal that binds to TSLP and blocks its interaction with its receptor complex | Moderate-to-severe asthma | IV every 2–4 weeks | Exacerbations FEV1, AHR, eosinophils response to allergens | Lacking data – reports of pneumonia, GBS, and stroke | Studies ever in mild asthma Not dependent on biomarkers such as blood AEC |
- 1.
Tezepelumab (AstraZeneca, Cambridge, UK)
Broadly speaking, these agents are cytokine antagonists that act by either binding/inhibiting circulating cytokines (e.g., omalizumab or mepolizumab) or by blocking binding to their receptors (e.g., benralizumab). They are administered either subcutaneously (e.g., omalizumab or mepolizumab) or intravenously (e.g., reslizumab). They have weight-based (e.g., omalizumab and reslizumab) or fixed dosing (e.g., mepolizumab and benralizumab) schemes. In general, these agents are well tolerated (adverse reactions in ~3% of patients), and severe reactions are rare (anaphylaxis in <1% of patients). Annual costs for these agents are influenced by several factors but vary from ~$10,000 to $34,000 per patient. These high costs limit the use of these agents mostly to developed parts of the world.
While biologics have been studied mostly in patients with moderate-to-severe asthma, most experts suggest their use be limited to refractory asthma [65]. Patients with refractory asthma are those for whom alternative diagnoses have been excluded, comorbidities have been treated, triggers have been removed, and compliance with treatment has been checked, but they remain poorly controlled and/or continue to experience frequent or severe exacerbations despite prescription of high-intensity treatment. An example would be Global Initiative for Asthma (GINA) Step 4–5 therapy or OCS for maintenance therapy (described as OCS use for ≥50% of the previous year) [66, 67]. “Difficult-to-control” asthma refers to patients in whom asthma control is sub-optimal due to adherence issues or comorbidities. While appealing, biologics should not be prescribed to these patients since control can be achieved in a good number of them by improving medication adherence or by correcting inhaler technique alone [68]. The therapeutic effects of biologics and their effect on clinical and biomarker-based outcomes are covered in this section and included in Table 8.1b. The mechanisms of action, patient selection characteristics, and expected outcomes for the currently available and pipeline biologic drugs in asthma have been summarized in Table 8.2.
8.7.1.1 Anti-IgE Therapy
Omalizumab was initially approved in 2003 and 2005 by the FDA and the European Medicines Agency (EMA), respectively, for patients aged 12 years and older. It was approved for pediatric patients aged 6–12 years in 2009 by the EMA, 2014 by UK’s NICE (National Institute for Health and Care Excellence), and 2016 by the FDA. It is a humanized IgG1 antibody that binds to the Cε3 domain of free IgE and prevents it from binding to its receptor, FcεR1. Treatment with omalizumab reduces unbound IgE capable of binding to its receptor by 90–95% [69–71] and subsequent downregulation of FcεR1 expression on basophils and mast cells [72] disrupting a key pathogenic step in allergic asthma.
Given the number of years it has been available, omalizumab is the most reviewed biologic in asthma [69, 73, 74] and probably the most studied. Early studies demonstrated its ability to inhibit the early and late asthmatic response to allergens including airway hyperresponsiveness to methacholine and post-exposure FEV1 reduction [75, 76]. In phase II studies (317 patients with moderate-to-severe asthma), omalizumab improved asthma symptom scores, decreased OCS dose in high-dose patients, improved peak expiratory flow rates (PEFR) and quality of life scores, and decreased exacerbations [70]. Similar data were seen in phase III studies involving adults [77–79] and children [80, 81]. In these studies, omalizumab was shown to significantly reduce exacerbations/hospitalizations, reduce ICS dose, improve FEV1, and improve asthma control. Of note, in the EXTRA study, the difference in exacerbations rates between omalizumab and placebo was highest in patients with a high T2 inflammatory profile (high FeNO, blood eosinophils, and serum periostin) [39]. Finally, in an eight-trial meta-analysis involving 3429 patients aged 5–79 years, omalizumab was associated with reduced exacerbation rates, ICS dose, and rescue medication requirements [82].
Omalizumab is indicated as add-on maintenance treatment for severe asthmatics with IgE levels of 30–700 IU/mL and allergic sensitization (demonstrated by skin testing or in vitro testing to seasonal aeroallergens). It is administered subcutaneously every 2 or 4 weeks based on age, body weight, and pre-treatment IgE levels (dose of 0.016 mg/kg per IU of IgE). A minimum duration of 12 weeks is currently recommended before determining the degree of clinical response [83, 84]. The long-term efficacy of this biologic has been demonstrated at least up to 40 months of treatment in clinical trials and anecdotally much longer [85]. Given its potential as a disease-modifying agent, there are no firm recommendations for stopping omalizumab in patients with a satisfactory clinical response. The most common adverse reaction is injection site reaction (45% of patients). Anaphylaxis (0.2% in post-marketing patient analysis) typically occurs within the first 60–90 minutes after injection but can last days after the injection. Prescribing information recommends monitoring of patients for a sufficient time after each injection. In clinical trials, malignancy was reported in 0.5% of patients treated with omalizumab versus 0.18% of patients treated with placebo, prompting concern over the drug that affected the pathogenesis of malignancy [86]. While pooled analysis of clinical data and a post-marketing safety study found no increased risk of malignancy, there was a small increase in cardiovascular and cerebrovascular events in patients on omalizumab that, while most likely attributable to differences of asthma severity in the study groups, will require close post-marketing attention for years to come [87, 88].
Other anti-IgE strategies failed to make it to phase III clinical trials. These include lumiliximab/IDEC-152 (depletes CD23b-producing cells) and quilizumab (an anti-M1’ antibody that targets IgE-producing cells) that decreased IgE levels but failed to improve clinical outcomes [89, 90]. Other agents were halted in development due to safety concerns for anaphylaxis risk in high-affinity anti-IgE agents [91]. CGP 51901, a chimeric anti-IgE antibody that binds to free and surface (IgE-expressing B cells) IgE, was shown to safely reduce IgE levels in patients with allergic rhinitis [92] and pollen sensitivity [93]. Unfortunately, further trials were stopped due to change in drug development priorities when Tanox collaborated with Genentech in favor of omalizumab [72].
8.7.1.2 Anti-IL-5 Therapy
IL-5 is a key cytokine in eosinophilic asthma and correlates with disease severity [17]. It promotes eosinophil differentiation and survival as well as airway eosinophil recruitment [94]. In pre-clinical models, anti-IL-5 antibody reduced both eosinophils in the circulation and bronchoalveolar fluid [19] making them attractive in the management of asthma. There are currently three FDA- and EMA-approved anti-IL-5 therapies. A recent meta-analysis concluded that these drugs halve the rate of exacerbations in patients with refractory eosinophilic asthma [95].
8.7.1.3 Mepolizumab
Mepolizumab was approved by the FDA in 2015. It is a recombinant, humanized IgG1 antibody that inhibits IL-5 from binding to the α-subunit of the IL-5 receptor complex expressed on eosinophils. Early studies with mepolizumab demonstrated a significant reduction in eosinophils but failed to demonstrate efficacy with relevant clinical outcomes [17, 18]. After these failures, future studies targeted patients with eosinophilic asthma only. The subsequent study with eosinophilic asthma patients (DREAM study) demonstrated that three different doses of intravenous mepolizumab resulted in a nearly 50% reduction in exacerbation frequency [20]. In addition, mepolizumab resulted in an improvement in quality of life and severe exacerbations during studies in the phase III program [96, 97]. There were two pivotal studies that led to FDA approval of mepolizumab in 2015. In the MENSA study, patients with refractory asthma (on high-dose ICS) were treated with placebo, intravenous, or subcutaneous mepolizumab [98]. Treatment groups had significant improvements in exacerbation rates (32% decrease in exacerbations requiring ED visits in the intravenous group and 61% in the subcutaneous group), lung function, asthma symptom control, and quality of life scores. In the SIRIUS study, patients with refractory asthma on oral corticosteroids had a median 50% reduction in their OCS dose, an improved asthma control, and a 32% annualized reduction in exacerbation rates [99]. Finally, a recent four-study meta-analysis of 1388 patients showed that mepolizumab halved exacerbations requiring hospitalization and/or emergency room visits compared to placebo [100].
Mepolizumab is indicated as add-on maintenance treatment for patients with severe eosinophilic asthma. It has been approved by the FDA (age >12 years) and the EMA (age >6 years) for management of severe eosinophilic asthma. Blood absolute eosinophil counts of ≥150 cells/μL at the time of screening or ≥300 cells/μL in the previous year were inclusion criteria for clinical trials. While the FDA, EMA, and GINA do not specify a blood eosinophil cutoff, NICE guidelines recommend a cutoff of ≥300 cells/μL. Mepolizumab is administered as a 100 mg subcutaneous dose every 4 weeks in patients ≥12 years. Adverse reactions including hypersensitivity reactions are generally seen within hours of administration. Adverse reactions that occurred more frequently than placebo (at least 3% of patients) include headache, injection site reaction, back pain, fatigue, influenza, urinary tract infection, upper abdominal pain, pruritis, eczema, and muscle spasms. There is a warning regarding herpes zoster infection, based on two serious adverse events in the clinical trials. Given the immunological role of eosinophils, the FDA has also required a warning about the risk of helminth infections. Once initiated, the duration for which therapy should be continued is unknown. Cessation is associated with a rise of blood eosinophils beginning soon after therapy is stopped; however, it remains unclear whether there will be lasting effects of mepolizumab on asthma control while patients are off therapy [101].
Finally, it is important to note that mepolizumab did not suppress sputum eosinophilia in ~50% of patients, and these patients tended to have a more modest reduction in exacerbation and steroid-sparing effects than those patients in whom sputum eosinophilia was suppressed [65]. This may be due to IL-5-driven in situ eosinophil production, compensatory effect of eosinophilopoietic cytokines produced locally within the tissue, or use of a lower dose of the drug [102]. Alternative strategies for these patients have not been explored and could involve higher dosing of mepolizumab, changing to weight-based dosing with reslizumab or receptor targeting with benralizumab. IL-4- and IL-13-mediated inflammation also results in eosinophilia and concomitant inflammatory pathways that would persist in the presence of upregulation of I L-5. It is likely that patients have more than one inflammatory pathway causing asthma.
8.7.1.4 Reslizumab
Reslizumab was FDA approved in 2016. It is a humanized IgG4 antibody that inhibits IL-5 from binding to the α-subunit of the IL-5 receptor complex expressed on eosinophils. As with mepolizumab, initial non-targeted pilot studies for reslizumab failed to demonstrate significant improvement in clinical outcomes while demonstrating eosinophil depletion [19]. The subsequent phase II study was focused on eosinophilic asthmatics only (sputum eosinophils ≥3%) and showed a reduction in sputum eosinophils with improvement in airway function and a trend toward better asthma control [21]. Subsequent phase III trials were launched as the BREATH program. This program resulted in three key publications from four studies [103–105] (two studies were presented in the same paper [104]). These studies revealed that reslizumab improved FEV1, improved asthma control, improved quality of life, and reduced exacerbation rates. Patients with frequent exacerbations and high blood eosinophil counts had the greatest benefit in terms of reduction of exacerbations [104] and FEV1 [105], respectively.
Reslizumab is indicated as add-on maintenance treatment for patients with severe eosinophilic asthma. Peripheral eosinophilia is defined as an absolute eosinophil count ≥400 cells/μL. Reslizumab is administered at a dose of 3 mg/kg by intravenous infusion over 20–50 minutes. Adverse reactions noted included anaphylaxis (0.3%) that was most often noted 20 minutes after the infusion. Malignancy was also reported more frequently with the reslizumab infusion (0.6%) than placebo (0.3%). Transient elevations of creatinine phosphokinase >10 times the upper limit of normal were noted in 0.8% of the treatment group compared to 0.4% of the placebo; but these increases were asymptomatic. Overall, reslizumab appears to be well tolerated. A long-term open-label extension study supported its safety and long-term efficacy f or at least 2 years [106].
8.7.1.5 Benralizumab
Benralizumab was FDA approved in 2017. It is a humanized afucosylated recombinant IgG1 antibody that binds with high affinity to the α-subunit of the IL-5 receptor that inhibits the proliferation and activation of eosinophils. Benralizumab also has increased affinity for heavy chain binding to the Fc receptor FcγRIIIα on natural killer cells which depletes existing eosinophils by inducing apoptosis through antibody-dependent cell-mediated cytotoxicity [107].
Phase I studies demonstrated benralizumab reduced airway, blood, and bone marrow eosinophils [108]. Phase II studies were associated with clinical efficacy with a reduction in exacerbations of 41% in eosinophilic asthma patients only [109]. The results of two phase III studies, CALIMA (moderate-to-severe asthma) [110] and SIROCCO [111], were published simultaneously. Both studies had similar results in terms of reduced exacerbation rates (with both every 4-week (Q4W) and 8-week dosing (Q8W)) and FEV1 improvement with benralizumab even in patients with eosinophils <300 cells/μL. The improvement in asthma symptom scores seen in CALIMA was not seen with Q4W regimen in SIROCCO. The ZONDA study was a 28-week trial where benralizumab demonstrated a 50% decrease OCS requirement compared to placebo with concomitant decreased exacerbation rates [112].
Benralizumab is indicated as add-on maintenance treatment for patients with severe eosinophilic asthma. Peripheral eosinophilia is defined as an absolute eosinophil count ≥300 cells/μL, albeit studies evaluating lower eosinophil cutoffs are ongoing (NCT03170271). It is given 30 mg every 4 weeks for the first three doses and then 30 mg every 8 weeks. Of note, unlike other biologics, benralizumab is supplied as a pre-filled autoinjector. Though speculative, this opens the possibility of self administration, obviating the need for office visits. The results of the GREGALE study were presented at the American Thoracic Society and demonstrated the potential for reliable self injections at home [113]. The most common adverse reactions noted were worsening asthma and nasopharyngitis. Hypersensitivity reactions (anaphylaxis, angioedema, and urticaria) occurred a higher rate (3%) than those reported with mepolizumab and resli zumab.
8.7.1.6 Dupilumab
Dupilumab is a fully human monoclonal IgG4 antibody to the alpha unit of IL-4 receptor. This receptor is a heterodimeric receptor complex that is activated by IL-4 and IL-13, key asthma cytokines produced by CD4+ Th2 cells and type 2 innate lymphoid cells. Its efficacy and safety were demonstrated in two phase II studies [114, 115], where it decreased exacerbations even in patients with eosinophil counts <300 cells/μL [115]. In a recent phase III study, the annual rates of exacerbation were decreased by 47.7% compared to placebo in both 200 mg and 300 mg every 2-week dosing groups [47]. There was also a significant improvement in FEV1, especially in patients with eosinophil counts >300 cells/μL. In another phase III study, glucocorticoid-dependent asthmatics had a significant reduction in their OCS dose (~70% vs 42%), decrease in severe exacerbation rate, and improvement in FEV1 compared to placebo [116]. In both these studies, transient eosinophilia (over 3000 cells/μL) was observed after the administration of dupilumab in ~1.2% of patients. Four patients had symptoms attributable to increased blood eosinophils. Anti-drug antibody was noted in 2–5% of patients but did not appear to affect efficacy. Dupilumab is FDA approved for patients >12 years of age with moderate-to-severe eosinophilic asthma or OCS-dependent asthma and for adults with atopic dermatitis [117]. The manufacturer recommends a loading dose of 400 mg (600 mg for OCS-dependent asthma or comorbid atopic dermatitis), followed by 200 mg maintenance dosing every 2 weeks (300 mg if higher loading dose used).
Lebrikizumab [2, 38, 118] and tralokinumab [119–121] are IgG4 monoclonal antibodies to IL-13 that have been studied and shown inconsistent results in terms of exacerbations, FEV1, and asthma symptom scores even with the use of biomarkers to improve patient selection.
8.8 Other Pipeline Biologics
TSLP is produced by the airway epithelium in response to antigen stimuli. It is elevated in the airways of patients with asthma and correlates with disease severity [122, 123]. Tezepelumab (AMG 157) is a fully human IgG2 monoclonal antibody that binds to TSLP and blocks its interaction with its receptor complex. In the first proof of concept study, tezepelumab administered as an intravenous infusion every 28 days attenutated the FEV1 responses to allergens in mild allergic asthma patients compared to placebo [124]. In a recent phase II study (PATHWAY), tezepelumab was given subcutaneously as low dose (70 mg) every 4 weeks, medium dose (210 mg) every 4 weeks, and high dose (280 mg) every 2 weeks to patients with moderate-to-severe asthma [125]. Compared to placebo all the three groups had a significant reduction in exacerbation rates (61%, 71%, and 66%, respectively) regardless of baseline eosinophil count as well as improvement in FEV1. Based on these findings, tezepelumab was granted breakthrough therapy designation by the FDA in September 2018. Two critical phase III studies, NAVIGATOR (NCT03347279) and SOURCE (NCT03406078), are ongoing.
With better understanding of asthma endotypes, new biologics continue to be developed and tested. For example, IL-33 is a key cytokine involved in allergic airway disease. Alongside ILC2s, IL-33 not only serves as a therapeutic target but also a potential biomarker of disease [126]. GSK3772847 is an IL-33 receptor antibody that is in phase II studies (NCT03207243). As with other newer biologics, it could revolutionalize the asthma landscape. With the introduction of these agents, clinicians will have access to more management options than ever before for asthma care, making cases that would have been easily deemed “refractory” in the past now amenable to therapy. They will also be faced with some tough decisions in terms of patient selection, new adverse effects (e.g., antibody development against biologics or the development of tolerance), cost containment, and long-term monitoring efficacy. Fortunately, the development of these drugs is being paralleled with robust research in terms of therapeutics and asthma pathogenesis.
8.9 Bronchial Thermoplasty
Bronchial thermoplasty (BT) is a device-based therapy that is indicated for patients with severe asthma that remain symptomatic despite adherence to high-dose ICS and long-acting bronchodilators including beta agonists (LABA) and muscarinic antagonists (LAMA) [127]. The procedure targets airway smooth muscle (ASM) , which plays a critical structural and immunomodulatory role in the airway and contributes to both exacerbations and chronic airway remodeling in asthma [128]. Airway remodeling occurs in response to developmental abnormalities, allergens, infectious agents, and environmental factors and may consist of airway wall thickening [129], sub-epithelial fibrosis, fibroblast hyperplasia, and increased ASM mass [130–135].

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


