Trial
Drug
Patients
Primary outcomes
Main conclusions
SOLVD
The SOLVD Investigators [55]
Enalapril vs placebo
6,797 patients with ejection fractions ≤ 0.35
Death from any cause, hospitalizations for heart failure
Enalapril reduced the incidence of heart failure and the rate of related hospitalizations in patients with asymptomatic left ventricular dysfunction. There was also a trend toward fewer deaths due to cardiovascular causes among the patients who received enalapril
CONSENSUS II
Swedberg et al. [51]
Enalapril vs placebo
6,090 patients with acute myocardial infarctions and blood pressure above 100/60 mmHg
Death due to any cause within 6 months
Enalapril therapy started within 24 h of the onset of acute myocardial infarction did not improve survival during the 180 days after infarction
AIRE
The AIRE Study Investigators [52]
Ramipril vs placebo
2,006 patients with heart failure after an acute myocardial infarction
All-cause mortality
Risk for death, severe/resistant heart failure, myocardial infarction, stroke
Mortality from all causes was significantly lower for patients randomised to receive ramipril. Analysis of prespecified secondary outcomes revealed a risk reduction of 19 %
TRACE
Kober et al. [25]
Trandolapril vs placebo
1,749 patients with left ventricular ejection fraction ≤ 0.35
All-cause mortality
Cardiovascular death, heart failure, recurrent myocardial infarction
Long-term treatment with trandolapril in patients with reduced left ventricular function soon after myocardial infarction significantly reduced the risk of overall mortality, mortality from cardiovascular causes, sudden death, and the development of severe heart failure
SAVE
Rutherford et al. [44]
Captopril vs placebo
2,231 patients after an acute myocardial infarction with asymptomatic left ventricular dysfunction
Composite of recurrent myocardial infarction, cardiac revascularization and hospitalization for unstable angina
Captopril reduced recurrence of myocardial infarction and the need for cardiac revascularization but had no influence on the rate of hospitalization with a discharge diagnosis of unstable angina
BENEDICT-B
Ruggenenti et al. [42]
Verapamil/trandolapril vs trandolapril
281 hypertensive type 2 diabetes patients with microalbuminuria
Persistent macroalbuminuria (albuminuria >200 μg/min in two consecutive visits). Treatment targets were systolic blood pressure/diastolic blood pressure less than 120/80 mmHg and HbA1C less than 7 %
Verapamil added on trandolapril did not improve renal or cardiovascular outcomes. Independent of verapamil, trandolapril normalized albuminuria in half of patients and this translated into significant cardioprotection
Further, the Studies of Left Ventricular Dysfunction (SOLVD) trial [55] and the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) [53] demonstrated the beneficial effect of enalapril in reducing the number of fatal and nonfatal cardiovascular events in patients with heart failure. The CONSENSUS II trial [51] showed no improvement in survival or reduction in the rate of recurrent myocardial infarction. However, it demonstrated that patients receiving enalapril were less likely to require a change in therapy for treatment of heart failure.
The Heart Outcomes Prevention Evaluation (HOPE) [63] in patients with high cardiovascular risk (vascular disease or diabetes plus 1 other cardiovascular risk factor), showed that treatment with ramipril 10 mg/day reduced the risk of cardiovascular death, myocardial infarction and stroke, compared with placebo. In patients with coronary artery disease, the EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease (EUROPA) [16] found that perindopril 8 mg/day reduced the risk for the composite end point of cardiovascular death, myocardial infarction and cardiac arrest compared with placebo.
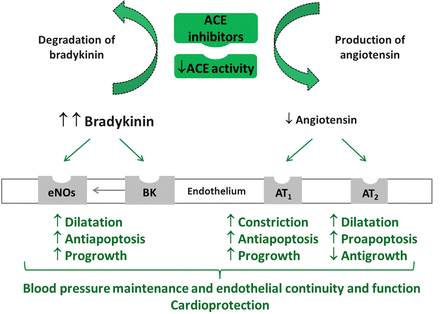
The efficacy of long-term ACEI monotherapy in reducing cardiovascular risk and protecting end-organ function and reducing cardiovascular events beyong blood pressure reduction is therefore well established, as recently reviewed [29]. Several other trials consistently indicated that ACE monotherapy improved cardiovascular outcomes in patients with hypertension or heart failure [25, 42, 52]. The mechanisms of action of ACE inhibition that may explain these results are shown in Fig. 4.1.
Monotherapy with ARBs
Table 4.2 shows the clinical trials that have evaluated the effect of ARBs on end-organ protection.
Table 4.2
Clinical trials with ARBs in hypertension or heart failure
Trial | Drug | Patients | Primary outcomes | Main conclusions |
|---|---|---|---|---|
TRANSCEND Yusuf et al. [64] | Telmisartan vs placebo | 5,927 ACEI intolerant patients with coronary, peripheral or cerebrovascular disease or diabetes with end-organ damage; controlled blood pressure | Composite endpoint of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke and hospitalization for congestive heart failure | In adults with vascular disease but without macroalbuminuria, the effects of telmisartan on major renal outcomes were similar to those of placebo |
I-PRESERVE Massie et al. [31] | Irbesartan vs placebo | 4,100 patients with chronic heart failure and relative preserved LV function | Composite outcome of death (all cause) and prespecified cardiovascular hospital admissions | Irbesartan did not improve the outcomes of patients with heart failure and a preserved left ventricular ejection fraction |
SCOPE Lithell et al. [28] | Candesartan vs placebo | 4,964 elderly patients | Composite outcome of cardiovascular death, non-fatal stroke and non-fatal myocardial infarction Secondary outcome measures included cardiovascular death, non-fatal and fatal stroke and myocardial infarction, cognitive function | A slightly more effective blood pressure reduction with candesartan was associated with a with a marked reduction in non-fatal stroke. Only a modest, statistically non-significant, reduction in major cardiovascular events was observed |
LIFE Dahlof et al. [9] | Losartan vs atenolol | 9,193 patients aged 55–80 years with essential hypertension and left ventricular hypertrophy | Composite of death, myocardial infarction and stroke | Losartan prevented more cardiovascular morbidity and death than atenolol for a similar reduction in blood pressure and was better tolerated |
VALUE Julius et al. [24] | Valsartan vs amlodipine | 15,245 patients with hypertension and high cardiovascular risk | Composite of cardiovascular death and cardiovascular events | The main outcome of cardiac disease did not differ between the treatment groups. Blood pressure was reduced by both treatments, but the effects of the amlodipine-based regimen were more pronounced, especially in the early period |
CHARM-Alternative Granger et al. [20] | Candesartan vs placebo | 2,028 patients with chronic heart failure, left ventricular dysfunction and ACEI intolerance | Composite of cardiovascular death and heart failure hospitalization | Candesartan was generally well tolerated and reduced cardiovascular mortality and morbidity |
Val-HeFT Cohn et al. [7] | Valsartan vs placebo added to standard therapy for heart failure | 5,010 patients with chronic heart failure | Combined endpoint of cardiovascular morbidity and mortality | Valsartan significantly reduced the combined end point and improved clinical signs and symptoms of heart failure, when added to prescribed therapy. However, when added to an ACEI or a beta-blocker the addition of valsartan had an adverse effect in terms mortality and morbidity |
MOSES Schrader et al. [49] | Eprosartan vs nitrendipine | 1,405 high-risk hypertensive patients with history of stroke | Composite of all-cause mortality, cardiovascular and cerebrovascular events | An early normotensive and comparable blood pressure was achieved. The combined end point was significantly lower in the eprosartan group |
However, several studies have failed to show non-inferiority of ARBs to ACEIs on hard end points such as death, doubling of creatinine or need for dialysis, and no placebo-controlled study has ever shown a protective effect of ARBs on hard end points. Two trials with hard end points, conducted in patients in advanced stages of diabetic nephropathy, the Reduction in End Points in Non-Insulin-Dependent Diabetes with the Angiotensin II Antagonist Losartan (RENAAL) study and the Irbesartan Diabetic Nephropathy Trial (IDNT), showed ARBs to provide benefits in patients with type 2 diabetes, but no benefit on cardiovascular outcomes was statistically significant. Thus, ARBs do not reduce cardiovascular events.
Two other studies – the Irbesartan Microalbuminuria Study (IRMA)-2 [36] and the Microalbuminuria Reduction with Valsartan study (MARVAL) [59] – showed the efficacy of ARBs in reducing microalbuminuria, a surrogate endpoint associated with early-stage diabetic nephropathy. Similarly, the Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) trial [64] found the efficacy of telmisartan on the following surrogate end points: serum creatinine, estimated glomerular filtration rate and albuminuria. These data are therefore not sufficient to draw conclusions on the efficacy of ARBs in reducing diabetic nephropathy. Indeed, whether the effect of ARBs on surrogate end points translates into a prognostic benefit is unknown.
Further, there is evidence from the Valsartan Heart Failure Trial (Val-HeFT) [7] that valsartan had a significant effect in reducing the mortality rate when added to standard therapy with heart failure, but not in the subgroup of patients taking an ACEI or a beta-blocker. In elderly patients with heart failure, the Irbesartan in heart failure with Preserved systolic function (I-PRESERVE) trial [31] studied the effect of irbesartan 300 mg/die on a primary composite outcome of death from any cause or hospitalisation for a cardiovascular cause. Secondary outcomes included death from heart failure or hospitalisation for heart failure, death from any cause and from cardiovascular causes, and quality of life. No significant differences in the outcomes were found. However, a subsequent sub-analysis of the trial [26] showed differential results for gender. Women (n = 2,491) with heart failure with preserved ejection fraction were more likely to have chronic kidney disease and hypertension than men. Thus, given these results, it may be relevant to assess the sex differences in risk among patients with heart failure.
Other ARBs, such as eprosartan [49] and candesartan [28], have been evaluated in clinical trials. The Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity (CHARM) trial [40] compared the effects of candesartan and placebo added to existing antihypertensive therapy in patients with chronic heart failure and left ventricular ejection fraction <40 %, finding that candesartan significantly reduced cardiovascular deaths and hospital admissions for heart failure. CHARM-Alternative [20] further evaluated candesartan therapy compared with placebo in patients who were intolerant to ACEIs, showing its effectiveness in reducing the incidence of cardiovascular death or hospital admission for heart failure. However, in patients with symptomatic heart failure but preserved systolic function in CHARM-Preserved [62], candesartan treatment was not associated with significant benefit.
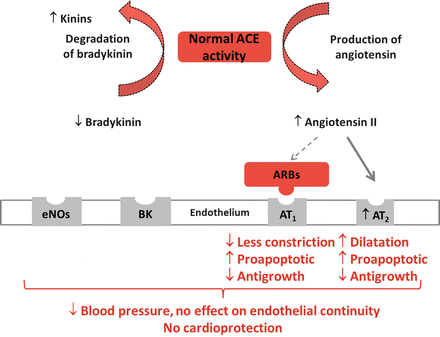
In conclusions, ARBs have been reported to have some beneficial effects only on surrogate end points associated with heart and kidney disease progression. There is evidence that ARBs may blunt progression of advanced diabetic nephropathy (see Fig. 4.2 for mechanisms of action), but their long-term renal effects in other patients are not clear.
Dual Therapy
Clinical trials have investigated the efficacy of dual therapy (ACEI + ARB) on cardiovascular and renal outcomes [2, 32, 34, 39]. Five trials have evaluated the effects of combination therapy with an ACEI and an ARB compared with treatment with either agent alone (Table 4.3).
Table 4.3
Clinical trials combining ACEI + ARB and clinical trials comparing ACEIs and ARBs in hypertension or heart failure
Trial | Drug | Patients | Primary outcomes | Main conclusions |
|---|---|---|---|---|
ONTARGET Yusuf et al. [64] | Telmisartan and ramipril both in monotherapy and combined | 23,400 high risk patients with coronary, peripheral or cerebrovascular disease or diabetes with end-organ damage; controlled BP | Composite endpoint of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke and hospitalization for congestive heart failure | In people at high vascular risk, the treatment effects on major renal outcomes were similar. Although combination therapy reduced proteinuria to a greater extent than monotherapy, overall it worsened major renal outcomes |
ELITE Pitt et al. [41] | Losartan vs captopril | 722 ACE-inhibitor naive patients aged ≥ 65 years with NYHA class II-IV heart failure and ejection fractions ≤40 % | Safety measure of renal dysfunction, composite of death and/or hospital admission for heart failure; and other efficacy measures | Treatment with losartan was associated with lower mortality than captopril. No difference in renal dysfunction was observed |
CHARM-Added | Candesartan vs placebo added to ACE inhibitor | 2,548 patients with heart failure and left ventricular ejection fraction ≤40 % | Composite of cardiovascular death and heart failure hospitalization | Candesartan reduced each of the components of the primary outcome significantly, as well as the total number of hospital admissions for heart failure. The benefits of candesartan were similar in all predefined subgroups, including patients receiving baseline beta blocker treatment |
VALIANT | Valsartan, captopril or both | 14,703 patients with acute myocardial infarction and heart failure, left ventricular dysfunction or both | All-cause mortality | Valsartan was as effective as captopril in patients at high risk for cardiovascular events after myocardial infarction. Combining valsartan with captopril increased the rate of adverse events without improving survival |
CALM Mogensen et al. [ 34] | Candesartan plus lisinopril vs candesartan and lisinopril alone | 199 patients with hypertension, type II diabetes, microalbuminuria | Change in blood pressure and UA:Cr ratio | Candesartan was as effective as lisinopril in reducing blood pressure and microalbuminuria in hypertensive patients with type 2 diabetes |
IMPROVE Bakris et al. [2] | Irbesartan ramipril or both | 405 patients with hypertension, microalbuminuria, high risk for cardiovascular events | Reduction in urinary albumin excretion | No differences between treatments for the endpoint. Although differences in blood pressure reductions were observed between the two treatments, these changes did not affect microalbuminuria. The incidence of adverse effects and treatment-related adverse effects was similar in both groups |
OPTIMAAL
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|