Fig. 5.1
EKG at rest while the patient was symptomatic
Routine Laboratory Tests
Complete blood count: normal
Renal function: creatinine 0.8 mg/dl (normal)
Hepatic function (GOT, GPT, y-GT, ALP, total bilirubin, direct and indirect): normal
Electrolytes: Na 137 mEq/L and K 4.1 mEq/L (normal)
Fasting blood glucose: 156 mg/dl
Troponin I: 0.13 ng/ml (n.v. <0.08 ng/ml)
CK-MB: 1.7 ng/ml (n.v. <5 ng/ml)
BNP 92 pg/ml (n.v. <100 pg/ml)
Inflammation index: VES 25 mm/h (n.v. <27 mm/h;) and CRP 0.5 mg/dl (n.v. <0.6 ng/ml)
D-dimers: 180 ng/ml (n.v. <230 ng/ml)
Chest X-Ray
Normal heart size and volumes. No bone fractures and no signs of trauma or dissection of the major mediastinal vessels. Absence of pulmonary congestion, pleural effusion, or signs of pneumothorax (not shown).
What Are the Possible Causes of Chest Pain in This Patient?
Myocardial disease
Acute coronary syndromes without persistent ST elevation (NSTE-ACS) or unstable angina (UA)
Myocardial infarction secondary to an ischemic imbalance for rise of blood pressure or tachycardia
Coronary spasm
Cardiac contusion
Stress cardiomyopathy (Takotsubo syndrome)
Myocarditis, pericarditis, and myopericarditis
Valvular heart disease
Severe aortic stenosis
Severe aortic regurgitation
Aortic disease
Traumatic aortic injury
Thoracic trauma and/or fractures
Pulmonary disease
Pneumothorax
Pulmonary embolism
A pulmonary embolism is unlikely for the low pretest probability (the patient has never suffered previous pulmonary embolism or deep vein thrombosis; she didn’t have previous surgery, trauma, long immobilization, or cancer; and she never used hormone replacement therapy). Furthermore, the normal D-dimer level excludes this affection with high sensitivity.
Pneumothorax and thoracic fractures can be excluded because physical examination and chest x-ray were normal.
The EKG shows ST segment depression in anterior leads which ruled out coronary spasm, usually characterized by ST elevation (Prinzmetal angina) although it may consider a possible posterior ST elevation with anterior ST depression as a mirror image.
A phlogistic damage (myocarditis, myopericarditis) is unlikely because the EKG modifications are not typical and not widely represented in all the EKG leads; moreover, the patient didn’t report flu or gastrointestinal disease, and the laboratory tests did not show clear signs of a phlogistic state.
The chest pain referred by the patient was quite typical for “angina” or also could suggest an aortic damage caused by car accident. An echocardiography was done to evaluate those possible diagnostic alternatives.
Echocardiography
The aortic valve is trileaflet. Slight enlargement of the left atrium (LA diameter M-mode = 4.2 cm; area 4 c = 22 cm2). Mild functional mitral regurgitation caused by displacement of the papillary muscles. Normal right atrium (area 4 c = 12 cm2). Right ventricle also normal in size and function. Normal tricuspid valve. Mild tricuspid regurgitation centrally directed (PASP = 35 mmHg). Normal pulmonic valve. The interatrial septum is normal. The left ventricle is slightly hypertrophic (LV mass index 97 g/m2) with apical and mid-left ventricular dilatation; moderate reduction of systolic global function for complete akinesis of the apical and mid-segments and compensatory basal hyperkinesis. The ejection fraction measured with Simpson’s biplane method was 40 %. Normal pericardium and aorta (aortic root dimension = 3.0 cm; ascending aorta = 3.2 cm; aortic arch = 3.3 cm; thoracic aorta = 2.9 cm; abdominal aorta = 2.0 cm). The inferior vena cava was not dilated. Pseudonormal diastolic pattern with increased filling pressure (E/A 1.1, E/E’ 16, E dec time 170 m/s) (Fig. 5.2).
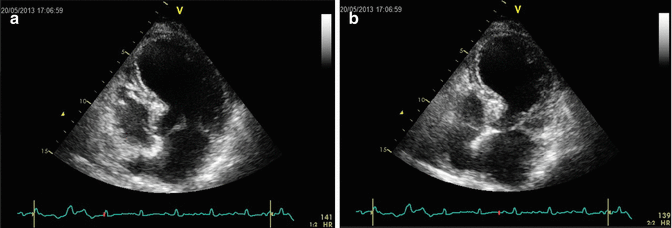

Fig. 5.2
Echocardiographic picture recorded at rest during systole (a) and during diastole (b); typical LV ballooning during systole is visible, characterized by complete akinesis in the apical to mid-segments circumferentially of the left ventricle with relative compensatory hypercontractility in the basal segments
Conclusion
Severe valvular disease, hypertrophic cardiomyopathy¸ pericardial effusion, and rupture of the wall or septum were ruled out.
Aortic root and aortic isthmus diameters were normal without any intimal flap or thrombus along the aortic wall. A traumatic aortic injury (TAI) was also unlikely because of features of the car accident suffered by the patient.
The echocardiography findings showed moderate reduction of systolic function for apical and mid-left ventricular dilatation and akinesia with compensatory basal hyperkinesis compatible with ischemic injury. This pattern of wall motion abnormality (“apical ballooning”) is quite typical for stress cardiomyopathy (Takotsubo-like), but also an AMI cannot be excluded without a coronary angiography.
While the patient has been prepared for coronary angiography, he has been treated with:
Initial loading dose of aspirin (300 mg)
Initial loading dose of ticagrelor (180 mg)
Fondaparinux 2.5 mg/day
Perindopril 2 mg/day
Atorvastatin 80 mg/day
Bisoprolol 2.5 mg/day
Coronary Angiography and Left Ventriculography
A coronary angiography was performed within 24 h from admission showing right coronary dominance and absence of significant coronary artery stenosis (Fig. 5.3).
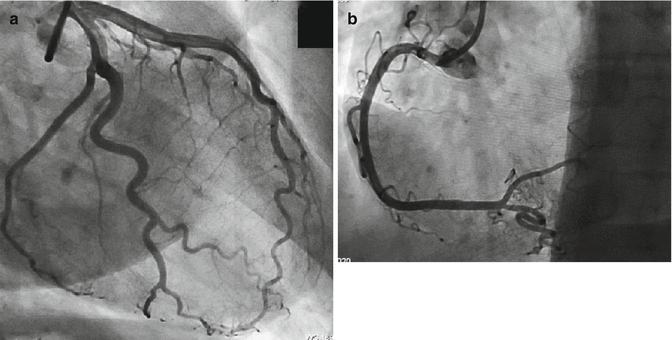

Fig. 5.3
Coronary angiography of left coronary artery (a) and right coronary artery (b); right coronary dominance and absence of significant coronary artery stenosis are shown
The left ventriculography showed complete apical and mid-ventricular akinesis with systolic “apical ballooning” and moderate systolic dysfunction. No spasm was recorded (Fig. 5.4).
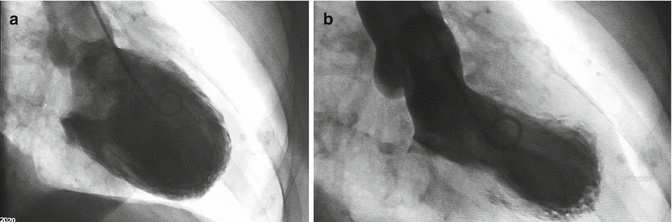

Fig. 5.4
Left ventriculography recorded during diastole (a) and systole (b); complete apical akinesis and mid-ventricular akinesis are shown, with systolic “apical ballooning” and moderate systolic dysfunction
Conclusion
Absence of significant culprit coronary artery stenosis or intracoronary thrombosis
“Apical ballooning” pattern
Final Diagnosis
The clinical presentation that mimics an acute myocardial infarction triggered by emotional stress (car accident) with modest EKG changes at presentation and with low plasma levels of cardiac biomarkers, disproportionate with the severity of ventricular dysfunction, could suggest the diagnosis of Takotsubo cardiomyopathy. This diagnosis is confirmed by findings on imaging (echocardiography and ventriculography) of transient apical- to mid-ventricular ballooning with compensatory basal hyperkinesis, without coronary stenosis.
Subsequent blood tests have shown normalization of cardiac biomarkers (max values: troponin I 2.45 ng/mL, CK MB 6.7 ng/mL), and echocardiography has displayed progressive recovery of contractile function until complete normalization.
Subsequent EKGs performed during hospitalization showed the typical evolution described in TC with new onset of negative and deep T waves and marked QTc interval prolongation and then slow and complete regression of these abnormalities after about 15 days.
EKG Performed at Day 3
Sinus rhythm at 65 bpm, normal atrioventricular conduction (PR 120 ms), complete right bundle branch block (QTS 140 ms), and normal ST segment with deep inverted T waves from V1 to V6 and DI, DII, AVL, and AVF (QTc interval of 624 ms) (Fig. 5.5).
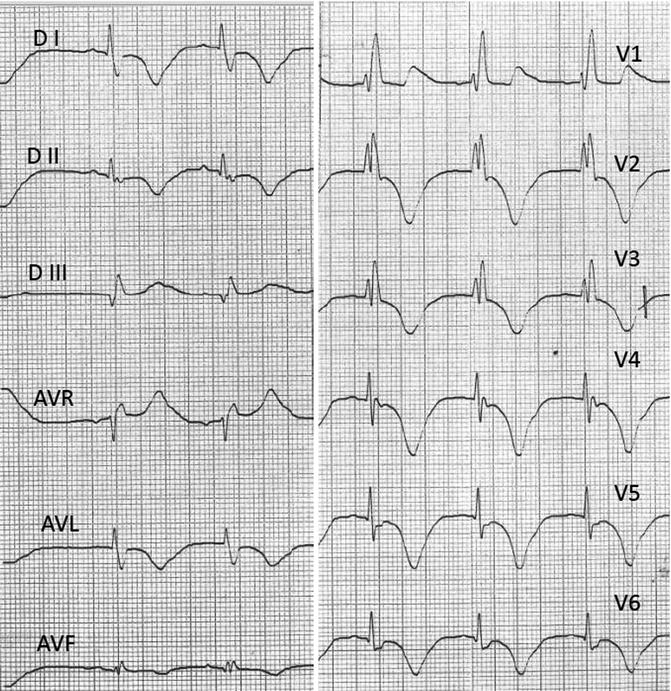

Fig. 5.5
EKG at rest during the third day of hospitalization while the patient was asymptomatic
The patient was discharged with the following therapy:
Aspirin 100 mg/day
Perindopril 2 mg/day
Atorvastatin 40 mg/day
Bisoprolol 5 mg/day
5.2 Takotsubo Syndrome
Definition
Takotsubo syndrome (TS) is a reversible cardiomyopathy, first described in 1990 by Sato et al. in Japan [1], also known as “apical ballooning syndrome” or “stress cardiomyopathy,” since it has become increasingly recognized worldwide.
It is characterized by signs and symptoms of acute myocardial infarction (AMI) but without demonstrable coronary artery stenosis.
Epidemiology
It typically occurs in postmenopausal elderly women (average age 68 years); men account for a minority of cases (4–13 %), and rarely also children or young adults may be affected [4].
Pathogenesis
The exact pathogenesis of Takotsubo cardiomyopathy (TC) is still unknown, but various hypotheses have been suggested and discussed over the years.
Coronary artery spasm or transient acute artery thrombosis [3, 5]: These hypotheses have been actually withdrawn because the typical “apical ballooning” is incongruent to a singular coronary artery supply region. The hypothesis of a multivessel coronary spasm would not explain the discrepancy between severe ventricular dysfunction and the only slightly increased levels of cardiac enzymes and why the recurrence could present different myocardial localization in the same patient or why some patients show the “apical sparing” variant [3].
The long-lasting ST segment elevation in Takotsubo patients challenges strongly the hypothesis of a transient thrombosis which usually presents rapid resolution of ST segment elevation [6].
Finally, the histological changes usually observed in TC (coagulative myocytolysis and myofibrillar degeneration) are different from those observed in ischemic disease (coagulative necrosis) [6].
Coronary microvascular dysfunction: This hypothesis has been initially proposed based on findings of a diminished coronary flow reserve [7] and on a significant improvement of myocardial perfusion during adenosine infusion in patients with TC but not with STEMI [8]. However, other trials didn’t confirm the “slow flow” phenomenon in many Takotsubo patients, so they supposed that this impairment of microcirculation could be a possible consequence of the myocardial dysfunction caused by TC itself [9].
Catecholamine toxicity: Some authors found markedly elevated catecholamine levels in patients with TC, suggesting that these might be the main pathogenic factor [10]; however, many subsequent studies have not uniformly shown elevated plasma catecholamine levels in TC patients [11]. So it is likely that circulating catecholamines are somehow involved in the pathogenic process but not the only ones responsible.
Cardiac sympathetic disruption: Recently a new hypothesis has been proposed regarding the disruption of local cardiac sympathetic nerve endings with local release of norepinephrine into the myocardial tissue, which could damage it and lead to the systolic dysfunction; moreover, it seems that the characteristic circular pattern of ventricular wall motion abnormality follows the cardiac sympathetic supply system [12].
Clinical Features
The clinical features of TS are usually completely superimposable with those of an acute myocardial infarction (AMI).




Symptoms: acute and prolonged anginal chest pain, described like retrosternal pressure or heaviness, sometimes radiating to the left arm or neck. When present, a typical aspect useful for diagnosis is that the chest pain usually occurs after mental or physical stress, such as the unexpected death of a relative or friend or receiving news of serious diagnosis or having a great fright [13]. However, recently an international meta-analysis (COUNTS study) has shown that these stressors have a limited impact occurring in about only 36 % of patients [14]. In men, physical stress rather than emotional stress is much more associated with the occurrence [4].< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


