Tachyarrhythmias
Omeed Zardkoohi
I. INTRODUCTION.
Tachyarrhythmias have been classically categorized by their location and mechanism. Tachyarrhythmias can originate from ventricular tissue (ventricular tachycardia) or, alternatively can originate from or involve supraventricular tissue (supraventricular tachycardia). The three mechanisms of tachyarrhythmias include abnormal automaticity, triggered activity, and reentry
A. Abnormal automaticity. Automaticity refers to the ability of cardiac tissue to spontaneously generate pacemaker activity. There are both normal and abnormal sources of automaticity.
1. An example of normal accelerated automaticity is the rapid firing rates of a normal pacemaker focus, such as the sinus node (SN), atrioventricular (AV) node, or Purkinje system due to ischemia, metabolic disturbance, exercise, or pharmacologic manipulation. A clinical example would be accelerated sinus tachycardia or junctional rhythm.
2. Abnormal automaticity refers to tissues that under normal circumstances do not demonstrate automaticity, but can become automatic in the setting of ischemia, metabolic disturbance, or pharmacologic manipulation. Overall, abnormal automaticity is responsible for < 10% of tachyarrhythmias. These latent or ectopic loci of cells generate automatic, spontaneous impulses that usurp control of the cardiac rhythm. These usually have a warm-up and cool-down period and cannot be induced by programmed electrical stimulation. A clinical example would be accelerated idioventricular rhythm (see Section IV.C.1) or multifocal atrial tachycardia (see Section II.F).
B. Triggered activity refers to pacemaker activity that is dependent on afterdepolarizations from a prior impulse or series of impulses. Afterdepolarizations are oscillations in the membrane potential. If these reach the critical threshold for depolarization of the surrounding cardiac tissue, they may trigger an action potential, thereby precipitating further afterdepolarizations and perpetuating the pacemaker activity. The two categories of afterdepolarizations are early and delayed.
1. Early afterdepolarizations (EADs) occur before repolarization of the cardiac tissue is completed (during phase 3 of the action potential) and may be the mechanism responsible for the ventricular arrhythmias of the long QT syndromes (LQTSs), as well as torsade de pointes (“twisting of the points”) produced by class I and class III antiarrhythmics, sympathetic discharge, and hypoxia. Antibiotics such as macrolides, certain azole antifungal agents, some psychotropic medications such as haloperidol, and several nonsedating antihistamines have been shown to produce EADs. Rapid heart rates and the administration of magnesium have been shown to suppress EADs.
2. Delayed afterdepolarizations (DADs) occur after the repolarization of the surrounding tissue is complete (during phase 4 of the action potential) and are thought to be the mechanism of triggered atrial tachycardia, arrhythmias of digitalis toxicity, and rare ventricular tachycardias (VTs) responsive to calcium channel blockers. These have been demonstrated in various cardiac issues, including
parts of the conducting system, myocardial cells, and valve tissues. Increases in intracellular calcium are associated with DADs, such as those caused by digitalis preparations or excessive sympathetic stimulation. Drugs that block the influx of calcium (such as calcium channel blockers and β-blockers) and drugs that decrease the sodium current (such as lidocaine and phenytoin) suppress the occurrence of DADs, whereas rapid heart rates augment DADs.
parts of the conducting system, myocardial cells, and valve tissues. Increases in intracellular calcium are associated with DADs, such as those caused by digitalis preparations or excessive sympathetic stimulation. Drugs that block the influx of calcium (such as calcium channel blockers and β-blockers) and drugs that decrease the sodium current (such as lidocaine and phenytoin) suppress the occurrence of DADs, whereas rapid heart rates augment DADs.
C. Reentry. Reentry is the most common cause of tachyarrhythmias. In order for reentry to occur, three conditions must be met:
1. Two functionally distinct conducting pathways must connect to form a circuit.
2. Unidirectional conduction block occurs in one of the pathways due to differences in refractory periods (block occurs in pathway with the longer refractory period).
3. Slow conduction occurs down the unblocked pathway (which has the shorter refractory period), allowing the blocked pathway time to recover excitability and sustain the arrhythmia.
Reentrant circuits can occur in the sinus node, the atrium, the AV node, between the atrium and ventricle via bypass tracts, and within the ventricle itself. The typical substrate for malignant reentry in the ventricle is scar or ischemia, which can produce regions in the heart that depolarize and repolarize heterogenously. Therefore, the impulse can spread to an area that has already repolarized after being previously depolarized. This can set up a circular movement of the impulse resulting in sustained tachyarrhythmias such as VT. Reentry can typically be induced by premature electrical stimulation during electrophysiologic testing.
Elucidation of the mechanisms of tachyarrhythmias has led to the development of catheter-based treatment strategies and more advanced medical therapy.
II. SUPRAVENTRICULAR TACHYARRHYTHMIAS
A. Sinus tachycardia
1. Clinical presentation. Sinus tachycardia manifests as sinus rhythm with a rate above 100 beats/min. Although the rate may be as high as 200 beats/min in younger individuals, it is generally 150 beats/min or less in older individuals.
2. Pathophysiology
a. The SN is an epicardial structure that is located laterally near the junction between the superior vena cava and the right atrium. Under normal circumstances, the rate of SN discharge is governed by sympathetic and vagal stimulation.
b. Sinus tachycardia generally reflects an underlying process, metabolic state, or effect of medication. Fever, hypovolemia, shock, congestive heart failure (CHF), anxiety, pulmonary disease including pulmonary embolism, anemia, thyrotoxicosis, caffeine, nicotine, atropine, catecholamines, or withdrawal from alcohol or drugs (both therapeutic and illicit) can cause sinus tachycardia.
c. Sinus tachycardia can be appropriate, where it represents a normal physiologic response, or inappropriate, as in defects in vagal or sympathetic tone or an intrinsic problem with the SN itself.
d. The clinical consequences of sinus tachycardia vary based on the presence or absence of underlying heart disease. Patients with significant coronary artery disease (CAD), left ventricular (LV) dysfunction, or valve disease may not tolerate sinus tachycardia. Patients with inappropriate sinus tachycardia may experience significant symptoms such as palpitations, dyspnea, and/or chest pain.
3. Diagnostic testing. Electrocardiography is the primary diagnostic test. The main differential is between sinus tachycardia, sinus node reentry tachycardia (SNRT) (see Section II.B), and inappropriate sinus tachycardia. Inappropriate sinus tachycardia is characterized by the following features: (a) heart rate > 100 beats/min, (b) P-wave axis and morphology during tachycardia similar or identical to that during sinus rhythm, (c) exclusion of secondary causes
of sinus tachycardia, (d) exclusion of atrial tachycardias, and (e) symptoms clearly documented to be related to resting or easily provoked sinus tachycardia.
of sinus tachycardia, (d) exclusion of atrial tachycardias, and (e) symptoms clearly documented to be related to resting or easily provoked sinus tachycardia.
4. Therapy is generally directed at the elimination of the underlying cause whenever possible.
a. If withdrawal from a therapeutic medication is suspected, then reinstitution or slow tapering of this medication can be attempted, if clinically appropriate.
b. In the case of inappropriate sinus tachycardia, β-blockers and calcium channel blockers may be necessary to control the heart rate.
c. In medically refractory cases, catheter ablation for sinoatrial nodal modification may have to be considered.
B. SNRT accounts for 5% to 10% of all supraventricular tachyarrhythmias.
1. Clinical presentation. SNRT is most frequently seen in patients with structural heart disease or CAD, especially in inferior myocardial infarctions (MIs). The rate varies from 80 to 200 beats/min. SNRT’s characteristic abrupt onset and termination (paroxysmal nature) along with its ability to be induced and terminated by pacing imply that the underlying mechanism is reentry and distinguish it from sinus tachycardia and inappropriate sinus tachycardia.
2. Pathophysiology. Reentry occurs within or adjacent to the SN and then conducts via the normal conduction pathway to the rest of the heart. The morphology of the P wave is identical to the underlying sinus morphology. Block at the AV node may occur, but it does not slow the tachycardia. In fact, a Wenckebachtype block often occurs with this rhythm. The development of a bundle branch block does not affect the cycle length or the PR interval.
3. Therapy. Vagal maneuvers or adenosine may successfully terminate this arrhythmia. Rapid atrial pacing can be used to induce and terminate this tachycardia. Various agents such as β-blockers, calcium channel blockers, and digoxin may help prevent recurrences. SN ablation or modification is rarely necessary.
C. Atrial fibrillation (AF) is the most common sustained arrhythmia, occurring in up to 1% of the general population. The prevalence of AF increases with age, affecting up to 10% of the population older than 80 years (see Chapter 24).
D. Atrial flutter. Atrial flutter is the second most common of the atrial tachyarrhythmias. Its reported incidence varies from 0.4% to 1.2% in hospital reports of electrocardiogram (ECG). The clinical significance of atrial flutter is generally due to its association with AF (with all of the attendant risks of AF) and/or its association with rapid rates of ventricular response.
1. Clinical presentation. The clinical presentation may vary widely depending on the presence of underlying heart disease, the ventricular rate, and the overall condition of the patient. It is occasionally reported to persist for days and, less commonly, for weeks or longer. Careful examination of the jugular venous pulse may reveal frequent, regular a waves that correspond to the atrial flutter rate. Like AF, it is commonly seen after open heart surgery, as well as with other conditions commonly associated with AF, such as pulmonary disease, thyrotoxicosis, atrial enlargement due to any cause including mitral/tricuspid valve disease, and SN dysfunction.
2. Pathophysiology. “Typical” atrial flutter is the result of a macroreentrant circuit in the right atrium. Atypical atrial flutter generally involves other macroreentrant circuits around scar tissue or surgical incisions.
a. In a typical atrial flutter, the reentrant circuit most commonly travels in a counterclockwise rotation down the right atrial anterolateral free wall across the cavotricuspid isthmus (area of slow conduction) and up the interatrial septum. Clockwise rotation of this circuit may also be seen.
b. Atrial flutter has been classified into type I and type II based on the following characteristics:
(1) Type I atrial flutter can be terminated with rapid atrial pacing and typically has an atrial rate in the range of 240 to 340 beats/min in the absence of drug therapy.
(2) Type II atrial flutter cannot be terminated with rapid atrial pacing and typically has an atrial rate in the range of 340 to 440 beats/min in the absence of drug therapy.
(3) Types I and II are not synonymous with typical and atypical atrial flutters. Type I atrial flutter can include typical and atypical atrial flutters. Type II atrial flutter is less well characterized than type I with respect to etiology and therapy; therefore, we refer to type I atrial flutter throughout this discussion.
3. Laboratory examination
a. The diagnosis can be difficult when the AV conduction is 2:1, as the flutter waves may be superimposed on the QRS complex and/or the T waves. When the diagnosis is uncertain, one should consider maneuvers or medications to slow the ventricular response, thus revealing the atrial flutter complexes.
(1) Vagal maneuvers include carotid sinus massage and Valsalva maneuver. Caution must be exercised when attempting carotid sinus massage in patients with known or suspected carotid disease or vagal maneuvers in patients with CAD who are at risk for ischemia.
(2) Adenosine can be administered, 6 mg rapid intravenous push, followed by 12 mg if there is no response (a second 12-mg dose can be given if there is no response). The half-life of this medication is very short, approximately 9 seconds. This causes transient (lasting seconds), complete AV block. Alternative agents include the intravenous calcium channel blocking agents verapamil and diltiazem and the intravenous β-blockers esmolol and metoprolol. Patients should be connected to a transcutaneous pacing device during the administration of this medication for reasons of safety.
(3) The clinician can place and record from a transesophageal electrode or record from a temporary atrial epicardial pacing wire (placed at open heart surgery). This results in an ECG with clearer atrial complexes and thus simplifies diagnosis. This strategy also allows a method of delivering rapid atrial pacing in an attempt to terminate the atrial flutter.
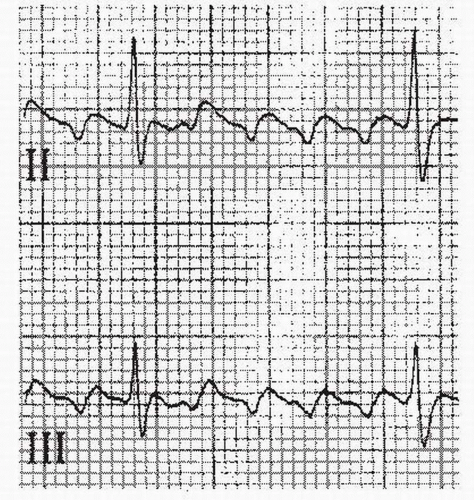
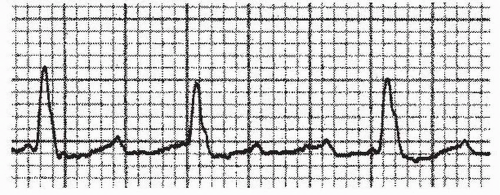
b. On the surface ECG, typical counterclockwise atrial flutter shows the classic negatively directed “sawtooth” waveform in the inferior leads (II, III, and aVF) (Fig. 21.1). Conversely, the atrial depolarizations are positive in these leads in clockwise atrial flutter (Fig. 21.2).
c. The atrial rate in the absence of drug therapy is 240 to 340 beats/min.
d. The QRS complex should be the same as that seen during sinus rhythm although aberrant conduction may occur, and the QRS may be slightly distorted by the atrial flutter waves.
e. The ventricular response can be irregularly irregular, due to varying degrees of block (2:1, 4:1, and so on), but is more typically regular as a fixed ratio of the flutter rate.
4. Therapy
a. Medical therapy differs very little from that for AF (see Chapter 24).
(1) Control of the ventricular response rate with a β-blocker, a calcium channel blocker, or digoxin is critical prior to initiating therapy with agents such as the class IA or IC agents. The class IA or IC agents either enhance AV nodal conduction through their vagolytic effects, thereby enabling 1:1 (AV) conduction, or slow the atrial rate to a point where 1:1 conduction is facilitated.
(2) The conversion from atrial flutter to AF after cardioversion is substantially reduced by the administration of antiarrhythmic drugs prior to direct current cardioversion (DCC), thereby increasing the chance of converting to sinus rhythm.
(3) Anticoagulation. There are no prospective data looking at the incidence of thromboembolic events with atrial flutter. However, retrospective data suggest an increased incidence of thromboembolic events. Recent ACCP (2004) and ACC/AHA/ESC (2006) guidelines recommend managing anticoagulation in atrial flutter in a manner similar to that for AF, including cardioversions. Optimal management is unclear and often needs to be individualized with the patients’ profile for thromboembolic risk dictating the type and duration of therapy. We treat atrial flutter in a manner similar to that used for AF with regard to anticoagulation.
b. Direct current cardioversion
(1) DCC is the preferred and most effective therapy for most patients. The procedure is detailed in Chapter 59. A starting energy as low as 25 to 50 J is often effective. Because DCC may result in conversion from atrial flutter to AF, a second shock is sometimes necessary to convert AF to sinus rhythm.
(2) Rapid atrial pacing should be considered as the first line of therapy for all patients who have epicardial atrial pacing wires in place after open heart surgery. It may be considered via a transesophageal pacing lead or via a transvenously placed pacing lead in patients for whom DCC fails or who are not candidates for DCC. Before attempting to rapidly pace the atria, it must be confirmed that ventricular capture is not inadvertently occurring by first pacing at a relatively slow rate while observing for such a phenomenon. Once this is confirmed, the atrium is paced at a rate of 10 to 20 beats/min faster than the underlying atrial flutter rate. Once atrial capture is attained, the rate is increased steadily until the hallmark negative-sawtooth waveform converts to a positive waveform. The pacing is then either halted abruptly or slowed rapidly to an acceptable atrial pacing rate. In cases that require extremely rapid rates of pacing (> 400 beats/min) or high amplitudes of pacing stimulus strength (> 20 mA), there is an increased tendency for the atrial flutter to convert to AF. When pacing via a transesophageal lead, a higher stimulus strength (up to 30 mA) may be necessary. Because this type of pacing can be quite painful, a sufficient energy to convert the atrial flutter should be used initially to minimize the conversion attempts.
(3) Percutaneous therapy. Radiofrequency ablation (RFA) of the cavotricuspid isthmus is often curative, with an efficacy > 90% for the long-term elimination of atrial flutter. Despite the high success rate of catheter-based therapy, a significant number of patients may subsequently develop AF.
E. Atrial tachycardias. This term encompasses a number of different types of tachycardias that originate in the atria. These tachycardias account for between10% and 15% of the tachycardias seen in older patients, usually in the setting of structural or ischemic heart disease, chronic obstructive pulmonary disease, electrolyte imbalances, or drug toxicity (particularly digitalis).
1. Clinical presentation. These tachycardias are infrequently seen in younger, healthy patients without underlying heart disease. They are typically paroxysmal, but if incessant they can lead to a tachycardia-induced cardiomyopathy.
2. Diagnostic testing
a. ECG
(1) The P-wave axis or morphology is usually different from that of sinus rhythm. One exception is atrial tachycardias originating from the right superior pulmonary vein, which is anatomically close to SN. The axis can be used to predict the origin of the atrial tachycardia.
(2) Atrial rhythm is regular, except with automatic atrial tachycardia, which displays a warm-up period (see Section II.E.3.b).
(3) A QRS complex that is generally identical to sinus rhythm (QRS can be wide if aberrant conduction occurs) follows each P wave.
(4) PR interval is within normal limits or prolonged.
(5) Nonspecific ST-T-wave changes may be present.
(6) When an AV block is present, there is an isoelectric baseline between P waves in all leads.
b. Electrophysiologic study has become critical in determining the underlying mechanism of these tachycardias, as the clinical differences are subtle and overlapping.
3. Subclassifications. The current subclassifications are based on mechanisms and include automatic atrial tachycardia, triggered atrial tachycardia, and intraatrial reentry.
a. Intra-atrial reentry is usually a disorder seen in those with underlying heart disease or history of atrial arrhythmia, such as AF or atrial flutter. The mechanism is not well understood. The ventricular rate is typically 90 to 120 beats/min due to the frequent occurrence of 2:1 AV block, such that hemodynamic effects are generally minimal. This rhythm can be difficult to distinguish from other supraventricular tachyarrhythmias. One clue is that despite any AV conduction block, the rhythm continues. The ability to terminate with adenosine and β-blockers is variable. RFA may be effective, with success rates > 75%. Antiarrhythmics (the same drugs as for AF and atrial flutter) have been disappointing in the prevention of recurrence.
b. Automatic atrial tachycardia appears to be generated by an ectopic atrial focus, which usually arises from regions around the crista terminalis in the right atrium and around the base of the pulmonary veins in the left atrium. The mechanism is not well understood. Automatic atrial tachycardia is seen more often in younger patients, displays a warm-up phenomenon (the supraventricular tachyarrhythmia accelerates after its initiation), does not respond to vagal maneuvers, and is more likely to be incessant. Automatic atrial tachycardia can be induced with treadmill testing or with administration of isoproterenol. Atrial stimulation during electrophysiologic study has no effect on either initiating or terminating this arrhythmia. Propranolol has been used successfully to suppress automatic atrial tachycardia. Catheter ablation is the preferred therapy when the tachycardia is incessant. Although adenosine may transiently slow automatic atrial tachycardia, it is unlikely to terminate it. Likewise, verapamil has been used without success.
c. Triggered atrial tachycardia is the least common of the atrial tachycardias and is virtually never incessant. It is more likely to appear in older individuals. It can be induced with rapid atrial pacing and is cycle length-dependent. The mechanism of triggered atrial tachycardia is thought to be due to DADs (see Section I.A.2) secondary to digitalis toxicity or sympathetic discharge. Catecholamines may play a role in the initiation of this arrhythmia, and thus exercise testing and isoproterenol may provoke it. Verapamil and adenosine have been shown to terminate triggered atrial tachycardia. β-Blockers have been less effective. RFA is preferred when the tachycardia is very symptomatic and not responsive to medication.
F. Multifocal atrial tachycardia
1. Clinical presentation. This atrial arrhythmia is uncommon and estimated to occur in 0.37% of hospitalized patients. The atrial rate is generally 100 to 130 beats/min. It occurs most often in elderly, critically ill patients and is frequently associated with concurrent pulmonary disease, particularly chronic obstructive pulmonary disease. It may also be seen in CHF and can degenerate into AF.
2. Pathogenesis and diagnostic tests. The mechanism appears to be abnormal automaticity or triggered activity arising from distinct atrial sites. The diagnosis requires the following criteria: (1) atrial rate > 100 beats/min, (2) P waves with three or more different morphologies, (3) varying P-P, P-R, and R-R intervals, and (4) the P waves separated by isoelectric intervals. Loss of AV conduction of each P wave is uncommon, making it possible to distinguish multifocal atrial tachycardia from AF.
3. Therapy is directed at the underlying illness, with little role for antiarrhythmics. Calcium channel blockers in high doses may be useful, or amiodarone when antiarrhythmic therapy is deemed necessary. Maintenance of electrolyte balance, particularly potassium and magnesium, may suppress the occurrence of multifocal atrial tachycardia.
G. Atrioventricular nodal reentrant tachycardia (AVNRT)
1. Clinical presentation. AVNRT usually has a narrow QRS complex with a ventricular rate typically in the range of 150 to 250 beats/min, although faster rates are infrequently observed. AVNRT is generally seen in patients without underlying heart disease. Palpitations and dyspnea are common presenting complaints. Angina, CHF, and rarely shock may be seen in those with a history of underlying heart disease. Syncope may occur due to rapid ventricular rates or due to asystole or bradycardia seen occasionally when this tachycardia terminates.
2. Pathophysiology. The mechanism in AVNRT appears to be a reentrant circuit composed of separate fast and slow atrial pathways involving the AV node. In 50% to 90% of patients with “typical” AVNRT, the antegrade conduction to the ventricles travels over the slow pathway and the retrograde conduction to the atria occurs over the fast pathway. The initiating event may be either a premature atrial complex (PAC) or a premature ventricular complex (PVC). The PAC blocks the fast pathway antegradely and conducts down the slow pathway then backs up the fast pathway after it has repolarized. Less commonly, a PVC conducts retrogradely to the atria via the fast pathway and then returns to the ventricles via the slow pathway. In the remaining 5% to 10% of patients, with atypical AVNRT, the antegrade conduction is down the fast pathway and retrograde via the slow pathway. The cycle length is thus dependent on the conduction velocity of the slow pathway, since the fast pathway generally has rapid conduction. Termination of the tachycardia is often the result of a block in the slow pathway. AV dissociation may develop during the tachycardia because the ventricles are not involved in the reentry circuit. This does not affect the rate of tachycardia nor does the development of bundle branch block.
3. Laboratory features and diagnosis. P waves are generally hidden within the QRS complex or at the terminal portion of the QRS in typical AVNRT. This may be visible as a small pseudo-R’ in lead V1 or small negative deflections in the inferior leads, as depolarization of the atria occurs simultaneously with ventricular depolarization. The RP segment is generally < 100 milliseconds. AVNRT is often induced abruptly by a PAC and its termination, which also tends to be abrupt, is often followed by a retrograde P wave. The termination may be followed by a brief period of asystole or bradycardia before the SN recovers from its tachycardiainduced suppression. The cycle length may vary, especially at the beginning and at the end of the tachycardia. This variation reflects the variable antegrade AV nodal conduction time. Vagal maneuvers may slow or terminate this tachycardia.
4. Therapy. Presently, the success and safety of percutaneous catheter ablation have allowed this approach to be considered equally with medical therapy as first-line therapy for long-term management of AVNRT. The decision about treatment approach should be individualized according to the characteristics of each patient and his or her arrhythmic patterns.
a. RFA has the advantage of curing the arrhythmia in the majority of instances and eliminating the need for long-term suppressive therapy with medications. Cure rates with catheter ablation for AVNRT are in excess of 95%.
b. Medical therapy. Medications that suppress AV nodal conduction such as β-blockers, calcium channel blockers, digoxin, and adenosine all slow or block conduction in the antegrade slow pathway, whereas class IA and class IC antiarrhythmic drugs slow the conduction in the retrograde fast pathway. Adenosine may be considered as first-line drug therapy for acute termination of AVNRT. This medication is available in an intravenous form only and has a very short half-life of about 9 seconds. The use of intravenous or oral β-blockers or calcium channel blockers is an alternative if adenosine is unsuccessful. The onset of action of digoxin limits its usefulness in terminating these arrhythmias, although it may be useful to prevent recurrences. Recurrences may be prevented in patients
with frequent sustained episodes with any of the above-mentioned agents except adenosine. Antiarrhythmic drug therapy is not routinely necessary or desirable for AVNRT, given the high success rates and low complication rates for catheter ablation.
with frequent sustained episodes with any of the above-mentioned agents except adenosine. Antiarrhythmic drug therapy is not routinely necessary or desirable for AVNRT, given the high success rates and low complication rates for catheter ablation.
c. DCC should be considered for patients whose disease is unstable or highly symptomatic. Low energies of 10 to 50 J are usually sufficient to terminate AVNRT.
H. Atrioventricular reentrant tachycardia (AVRT)
1. Clinical presentation. Similar to AVNRT, this is another example of an AV nodal-dependent supraventricular tachycardia (SVT). AVRT usually has a narrow QRS with ventricular rates similar to those of AVNRT, although it more often tends to have a ventricular rate > 200 beats/minute. The clinical features are very similar to those of AVNRT but are distinct on an electrophysiologic basis.
2. Pathophysiology. The mechanism in AVRT relies on the presence of an accessory pathway as one portion of the circuit and the AV node as the other portion. The atrium and the ventricle on the same side as the accessory pathway are necessary components of the circuit. AVRT may be orthodromic or antidromic. Orthodromic AVRT usually has a narrow complex that uses the AV node as the antegrade limb and the accessory pathway as the retrograde limb of the circuit. Antidromic AVRT has a wide complex that is the opposite of the orthodromic variety, such that the accessory pathway serves as the antegrade limb and the AV node as the retrograde limb of the circuit. AVRT is most often of the orthodromic type. Accessory pathways may be “concealed” (inapparent by ECG) because of having only retrograde (V to A) conduction properties or “manifest” (apparent on ECG as delta waves, i.e., Wolff-Parkinson-White [WPW] pattern). Unlike AVNRT, the AVRT circuit must involve one of the ventricles; therefore, the development of bundle branch block on the side ipsilateral to the accessory pathway can prolong the ventricular to atrial conduction time and often the cycle length of the tachycardia. Bundle branch block, particularly left bundle branch block (LBBB), occurs more commonly in AVRT than in AVNRT. AVRT can be distinguished from AVNRT by electrophysiologic study. The presence of AV or ventriculoatrial (VA) block with continuation of the tachycardia should exclude the presence of an accessory AV pathway.
3. Laboratory features and diagnosis. The P waves of AVRT are frequently inscribed on the ST segment or T wave, as the atrial depolarization and ventricular depolarization are in series rather than in parallel. The RP segment is generally > 100 milliseconds. Orthodromic AVRT is more common, accounting for about 95% of all AVRTs, whereas antidromic AVRT accounts for only about 5%. Orthodromic AVRT is usually characterized by a narrow QRS complex as opposed to antidromic AVRT, which is characterized by a wide QRS complex.
4. Therapy. See the discussion of therapy for WPW syndrome (Section I.4).
I. Preexcitation syndromes. Preexcitation was originally used to describe the premature activation of ventricle in patients with WPW. The term has broadened to include all conditions in which antegrade ventricular activation or retrograde atrial activation occurs partially or totally via an anomalous pathway distinct from the normal cardiac conduction system. The incidence of preexcitation on ECG is approximately 1.5 per 1,000 cases, most of which occur in otherwise healthy subjects without organic heart disease. About 7% to 10% of these patients have associated Ebstein’s anomaly and are thus more likely to have multiple accessory pathways. There is a higher rate of preexcitation in males, with the prevalence decreasing with age, although the frequency of paroxysmal tachycardia increases with age.
1. Clinical presentation. Approximately 50% to 60% of patients with preexcitation report symptoms such as palpitations, anxiety, dyspnea, chest pain or tightness, and syncope. In approximately 25% of the cases, the disease will
become asymptomatic over time. Those patients older than 40 years whose disease has been asymptomatic are likely to remain symptom free. The absence of preexcitation on ECG despite the discovery of accessory pathways in patients with asymptomatic disease likely identifies a group of patients at low risk for developing symptoms.
become asymptomatic over time. Those patients older than 40 years whose disease has been asymptomatic are likely to remain symptom free. The absence of preexcitation on ECG despite the discovery of accessory pathways in patients with asymptomatic disease likely identifies a group of patients at low risk for developing symptoms.
2. Pathophysiology. Patients with preexcitation generally have an accessory pathway(s) that alters the conduction between the atria and the ventricles. These accessory pathways are likely congenital, as relatives of subjects with preexcitation have an increased incidence of preexcitation. AVRT is the most common mechanism associated with preexcitation (80% to 85%), with permanent junctional reciprocating tachycardia, Mahaim fiber tachycardia, and Lown-Ganong-Levine (LGL) syndrome accounting for the remainder.
a. WPW syndrome. The basic abnormality lies in the existence of an accessory pathway of conducting tissue, outside of the normal conducting system, which connects the atria and the ventricles. This accessory pathway permits the atrial impulse to bypass the normal pathway through the AV node to the ventricles. In the past, these accessory pathways have been referred to as “bundles of Kent.” An impulse from the atria can be conducted down both the accessory pathway and the AV node, arriving at the ventricle at nearly the same time. This results in preexcitation of the ventricle, which is really a fusion beat, as a portion of the ventricle is activated via the accessory pathway (giving rise to the delta wave; Fig. 21.3) and the remainder of the ventricle is activated by the normal activation pathway. If antegrade conduction occurs exclusively via the accessory pathway, the resultant QRS is maximally preexcited and is a wide complex. These accessory pathways may conduct rapidly, but frequently have longer refractory periods than the AV node. The inciting event for AVRT is frequently a PAC that is blocked in the accessory pathway and that conducts to the ventricles via the AV node, which has recovered more rapidly. The resultant QRS complex in this instance is normal in appearance. After the QRS complex, the accessory pathway has had sufficient time to recover excitability, and the impulse thus conducts retrogradely to the atria. A small but significant percentage (5% to 10%) of patients have multiple accessory pathways.
b. Permanent junctional reciprocating tachycardia is a variant of AVRT. It is often an incessant supraventricular tachyarrhythmia with an unusual accessory pathway. Here, the accessory pathway behaves like the AV node in that it displays decremental retrograde conduction properties. Thus, the faster the stimulation of such an accessory pathway, the slower the conduction through the pathway. The accessory pathway is most often located in the posteroseptal region and acts as the retrograde limb of the reentrant circuit. The VA conduction is slowed by the decremental nature of the accessory pathway. Due to the incessant nature of this tachycardia, a tachycardiainduced cardiomyopathy may result.
c. Mahaim fiber tachycardias are another variant of reentrant tachycardia. The two most common varieties that are recognized are atriofascicular and fasciculoventricular. In the former, the accessory pathway is located within a few centimeters of the AV node and inserts into the right bundle branch. The reentrant tachycardia conducts antegrade via the accessory pathway, resulting in an LBBB morphology with left-axis deviation. The retrograde circuit is via the AV node. In the second form of Mahaim reentry, the accessory pathway arises in the His-Purkinje fibers and allows bypass of the distal conducting system.