Tetralogy of Fallot
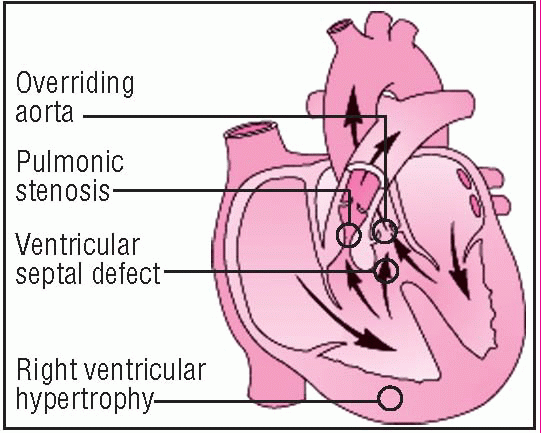
Tetralogy of Fallot is a combination of four cardiac defects: ventricular septal defect (VSD), right ventricular outflow tract obstruction (pulmonic stenosis), right ventricular hypertrophy, and dextroposition of the aorta, with overriding of the VSD. (See
Defects in tetralogy of Fallot, page 192.)
Blood shunts right to left through the VSD, permitting unoxygenated blood to mix with oxygenated blood, resulting in cyanosis. Tetralogy of Fallot sometimes coexists with other congenital heart defects, such as patent ductus arteriosus or atrial septal defect.
CAUSES AND INCIDENCE
The cause of tetralogy of Fallot is unknown, but it results from embryologic hypoplasia of the outflow tract of the right ventricle. Multiple factors, such as Down syndrome, have been linked to it. Prenatal risk factors include maternal rubella or other viral illnesses, poor prenatal nutrition, maternal alcoholism, mother older than age 40, and diabetes.
In tetralogy of Fallot, unoxygenated venous blood returning to the right side of the heart may pass through the VSD to the left ventricle, bypassing the lungs, or it may enter the pulmonary artery, depending on the extent of the pulmonic stenosis. Rather than originating from the left ventricle, the aorta overrides both ventricles.
The VSD usually lies in the outflow tract of the right ventricle and is generally large enough to permit equalization of right and left ventricular pressures. However, the ration of systemic vascular resistance to pulmonic stenosis affects the direction and magnitude of shunt flow across the VSD. Severe obstruction of right ventricular outflow produces a right-to-left shunt, causing decreased systemic arterial oxygen saturation, cyanosis, reduced pulmonary blood flow, and hypoplasia of the entire pulmonary vasculature. Right ventricular hypertrophy develops in response to the extra force needed to push blood into the stenotic pulmonary artery. Milder forms of pulmonic stenosis result in a left-to-right shunt or no shunt at all.
Tetralogy of Fallot occurs in about 5 of every 10,000 infants and accounts for about 10% of all congenital heart diseases. It occurs equally in boys and girls. Before surgical advances made correction possible, about one-third of these children died in infancy.
SIGNS AND SYMPTOMS
The degree of pulmonary stenosis, interacting with the VSD’s size and location, determines the clinical and hemodynamic effects of this complex defect. Generally, the hallmark of the disorder is cyanosis, which usually becomes evident within several months after birth but may be present at birth if the neonate has severe pulmonary stenosis. Between ages 2 months and 2 years, children with tetralogy of Fallot may experience cyanotic or “blue” spells. Such spells result from increased right-to-left shunting, possibly caused by spasm of the right ventricular outflow tract, increased systemic venous return, or decreased systemic arterial resistance.
Exercise, crying, straining, infection, or fever can precipitate blue spells. They’re characterized by dyspnea; deep, sighing respirations; bradycardia; fainting; seizures; and loss of consciousness. Older children may develop additional signs of poor oxygenation, such as clubbing, diminished exercise tolerance, increasing dyspnea on exertion, growth retardation, and eating difficulties. These children habitually squat when they feel short of breath; this is thought to decrease venous return of unoxygenated blood from the legs and increase systemic arterial resistance.
Auscultation reveals a loud systolic heart murmur (best heard along the left sternal border), which may diminish or obscure the pulmonic component of S2. In a patient with a large patent ductus, the continuous murmur of the ductus obscures the systolic murmur. Palpation may reveal a cardiac thrill at the left sternal border and an obvious right ventricular impulse. The inferior sternum appears prominent.
Children with tetralogy of Fallot also risk developing cerebral abscesses, pulmonary thrombosis, venous thrombosis or cerebral embolism, and infective endocarditis.
In females with tetralogy of Fallot who live to childbearing age, incidence of spontaneous abortion,
premature births, and low birth weight rises.
COMPLICATIONS
• Cerebral abscess
• Pulmonary thrombosis
• Venous thrombosis
• Cerebral embolism
• Infective endocarditis
DIAGNOSIS
• Chest X-ray may demonstrate decreased pulmonary vascular marking, depending on the pulmonary obstruction’s severity, and a boot-shaped cardiac silhouette.
• Electrocardiography shows right ventricular hypertrophy, right axis deviation and, possibly, right atrial hypertrophy.
• Echocardiography identifies septal overriding of the aorta, the VSD, and pulmonary stenosis and detects the hypertrophied walls of the right ventricle.
• Laboratory findings reveal diminished arterial oxygen saturation and polycythemia (hematocrit may be more than 60%) if the cyanosis is severe and long-standing, predisposing the patient to thrombosis.
• Cardiac catheterization confirms the diagnosis by visualizing pulmonary stenosis, the VSD, and the overriding aorta and ruling out other cyanotic heart defects. This test also measures the degree of oxygen saturation in aortic blood.
TREATMENT
Effective management of tetralogy of Fallot requires prevention and treatment of complications, measures to relieve cyanosis, and palliative or corrective surgery. During cyanotic spells, the knee-chest position and administration of oxygen and morphine improve oxygenation.
Palliative surgery is performed on infants with potentially fatal hypoxic spells. The goal of surgery is to enhance blood flow to the lungs to reduce hypoxia; this is often accomplished by joining the subclavian artery to the pulmonary artery (Blalock-Taussig procedure). Management may also include phlebotomy in children with polycythemia.
Complete corrective surgery to relieve pulmonary stenosis and close the VSD, directing left ventricular outflow to the aorta, requires cardiopulmonary bypass with hypothermia to decrease oxygen utilization during surgery, especially in young children. An infant may have this corrective surgery without prior palliative surgery. It’s usually done when progressive hypoxia and polycythemia impair the quality of his life, rather than at a specific age. However, most children require surgery before they reach school age.
Drugs
• Propranolol (Inderal)—a beta-adrenergic blocker—which may prevent blue spells
• Prophylactic antibiotics to prevent infective endocarditis or cerebral abscess administered before, during, and after bowel, bladder, or any other surgery or dental treatments
SPECIAL CONSIDERATIONS
• Explain tetralogy of Fallot to parents. Explain that their child will set his own exercise limits and will know when to rest. Make sure they understand that their child can engage in physical activity, and advise them not to be overprotective.
• Teach parents to recognize serious hypoxic spells, which can dramatically increase cyanosis; deep, sighing respirations; and loss of consciousness. Tell them to place their child in the knee-chest position and to report such spells immediately. Emergency treatment may be necessary.
• Instruct parents on ways to prevent overexerting their child, such as feeding him slowly and providing smaller and more frequent meals. Tell them that remaining calm may decrease his anxiety and that anticipating his needs may minimize crying. Urge parents to recruit other family members in the child’s care to help prevent their own exhaustion.
• To prevent infective endocarditis and other infections, warn parents to keep their child away from people with infections. Urge them to maintain the child’s dental hygiene, and tell them to watch for ear, nose, and throat infections and dental caries, all of which require immediate treatment. When dental care, infections, or surgery requires prophylactic antibiotics, tell the parents to make sure the child completes the prescribed regimen.
• If the child needs medical attention for an unrelated problem, advise parents to immediately inform the practitioner of the child’s history of tetralogy of Fallot because any treatment must take this serious heart defect into consideration.
• During hospitalization, alert the staff to the child’s condition. Because of the right-to-left shunt through the VSD, treat I.V. lines like arterial lines. A clot dislodged from a catheter tip in a vein can cross the VSD and cause cerebral embolism. The same thing can happen if air enters the venous lines.
After palliative surgery
• Monitor oxygenation and arterial blood gas (ABG) values closely in the intensive care unit.
• If the child has had the Blalock-Taussig procedure, don’t use the arm on the operative side for checking blood pressure, inserting I.V. lines, or drawing blood samples because blood perfusion on this side diminishes greatly until collateral circulation develops. Note this on the child’s chart and at his bedside.
After corrective surgery
• Watch for right bundle-branch block or more serious disturbances of atrioventricular conduction and for ventricular ectopic beats.
• Be alert for other postoperative complications, such as bleeding, right-sided heart failure, and respiratory failure. After surgery, transient heart failure is common and may require treatment with digoxin (Lanoxin) and diuretics.
• Monitor left atrial pressure directly. A pulmonary artery catheter may
also be used to check central venous and pulmonary artery pressures.
• Frequently check color and vital signs. Obtain ABG measurements regularly to assess oxygenation. Suction to prevent atelectasis and pneumonia, as needed. Monitor mechanical ventilation.
• Monitor and record intake and output accurately.
• If atrioventricular block develops with a low heart rate, a temporary external pacemaker may be necessary.
• If blood pressure or cardiac output is inadequate, catecholamines may be ordered by continuous I.V. infusion. To decrease left ventricular workload, give nitroprusside (Nitropress), if ordered, and provide analgesics as needed.
• Keep parents informed about their child’s progress. After discharge, the child may need digoxin, diuretics, and other drugs. Stress the importance of complying with the prescribed regimen and make sure the parents know how and when to administer these medications. Teach parents to watch for signs and symptoms of digoxin toxicity (anorexia, nausea, vomiting). Prophylactic antibiotics to prevent infective endocarditis will still be needed. Advise parents to avoid becoming overprotective as the child’s tolerance of physical activity rises.
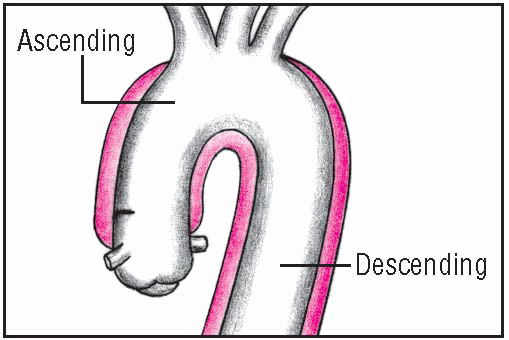
Thoracic aortic aneurysm
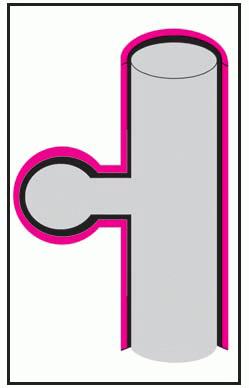
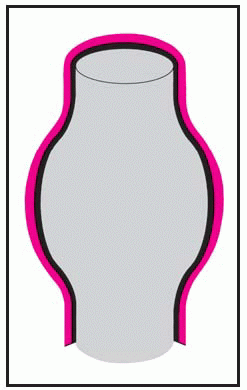
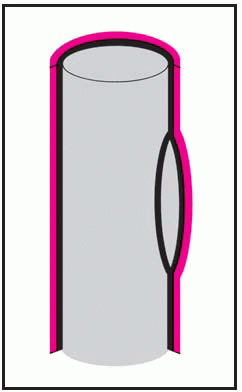
Thoracic aortic aneurysm is an abnormal widening of the ascending, transverse, or descending part of the aorta. Aneurysm of the ascending aorta is the most common type and has the highest mortality. Aneurysms may be dissecting, saccular, or fusiform. (See
Types of aortic aneurysms, page 196.) Some aneurysms progress to serious or, eventually, lethal complications, such as rupture of an untreated thoracic dissecting aneurysm into the pericardium with resulting tamponade.
CAUSES AND INCIDENCE
Thoracic aortic aneurysms commonly result from atherosclerosis, which weakens the aortic wall and gradually distends the lumen. An intimal tear in the ascending aorta initiates dissecting aneurysm in about 60% of affected patients. Regardless of the cause, these aneurysms affect 6 of every 100,000 people.
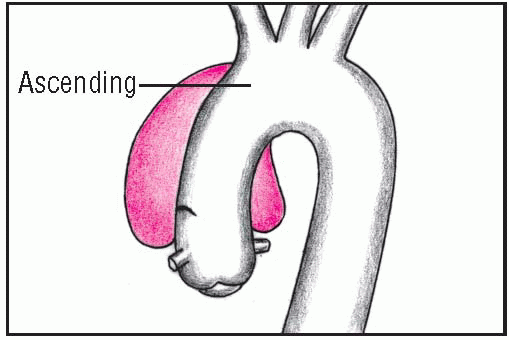
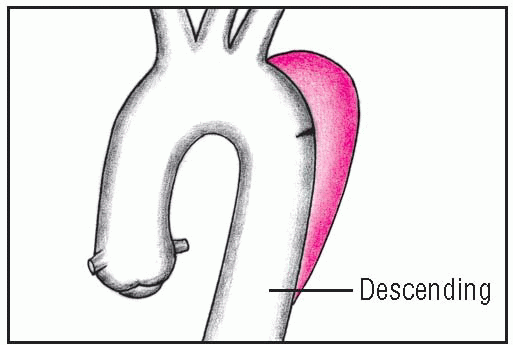
Ascending aortic aneurysms, the most common type, usually occur in hypertensive men younger than age 60. Descending aortic aneurysms, usually found just below the origin of the subclavian artery, are most common in elderly hypertensive men.
Descending aortic aneurysms are also seen in younger patients with a history of traumatic chest injury, less often in those with infection. Transverse aortic aneurysms are the least common type.
Other causes include:
• fungal infection (mycotic aneurysms) of the aortic arch and descending segments
• congenital disorders, such as coarctation of the aorta and Marfan syndrome
• trauma, usually of the descending thoracic aorta, from an accident that shears the aorta transversely (acceleration-deceleration injuries)
• syphilis, usually of the ascending aorta (uncommon because of antibiotics)
• hypertension (in dissecting aneurysm).
SIGNS AND SYMPTOMS
The most common symptom of thoracic aortic aneurysm is pain. With ascending aneurysm, the pain is described as severe, boring, and ripping and extends to the neck, shoulders, lower back, or abdomen but seldom radiates to the jaw and arms. Pain is more severe on the right side.
Other signs of ascending aneurysm may include bradycardia, aortic insufficiency, pericardial friction rub caused by a hemopericardium, unequal intensities of the right carotid and left radial pulses, and a difference in blood pressure between the right and left arms. These signs are absent in descending aneurysm. If dissection involves the carotids, an abrupt onset of neurologic deficits may occur.
With descending aneurysm, pain usually starts suddenly between the shoulder blades and may radiate to the chest; it’s described as sharp and tearing. Transverse aneurysm
causes a sudden, sharp, tearing pain radiating to the shoulders. It also may cause hoarseness, dyspnea, dysphagia, and a dry cough because of compression of surrounding structures in this area. (See
Classifying aortic dissection and
Clinical characteristics of thoracic dissection, page 198.)
COMPLICATIONS
• Rupture and hemorrhage
• Cardiac tamponade
• Superior vena cava obstruction (ascending)