Ronald G. Victor, Peter Libby
Systemic Hypertension
Management
Hypertension remains the most common diagnosis in adult outpatient medicine and the most common indication for prescription drugs. Lifestyle modification, particularly at the societal level, can prevent or delay the development of hypertension. Yet hypertension is becoming more prevalent in both developed and developing countries and remains poorly controlled in the United States and abroad.1,2
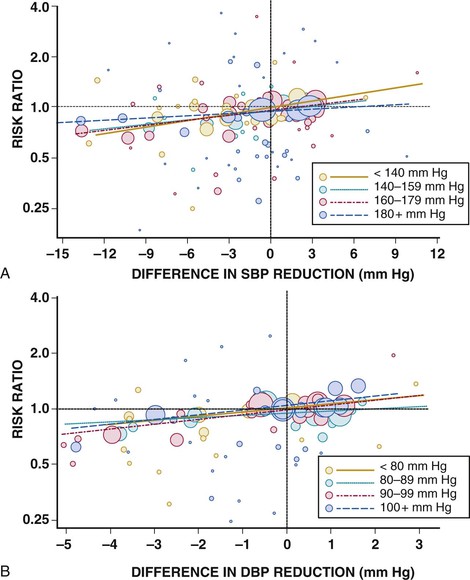
Reductions in high blood pressure (BP) lead to large reductions in the risk for stroke, heart failure, renal failure, aortic dissection, coronary events, and death. These benefits apply to all hypertensive patient groups regardless of age, race/ethnicity, sex, or severity of hypertension (Fig. 44-1). Except for some cases of secondary hypertension, most cases of hypertension cannot be cured. Yet many tools permit management of hypertension: lifestyle modifications, antihypertensive drugs, and now possibly cardiovascular (CV) interventions such as renal denervation. We will discuss their deployment based on the available evidence. Then, because of the recent release of different sets of hypertension guidelines both in the United States and abroad,3–12 we provide a practical clinical approach to the management of hypertensive patients.
Lifestyle Modification
Lifestyle choices and interventions can influence BP and furnish a foundation for prevention and treatment of hypertension. The current evidence base regarding dietary patterns and specific dietary components has sufficient strength to merit recommendations both on a population, public health level and on the management of individual patients. Evidence regarding physical activity interventions has lagged behind the evidence base on dietary approaches to the treatment of hypertension. Limitations regarding lifestyle and BP management require consideration. First, few studies have examined the effects of lifestyle interventions on CV outcomes; most rely on BP as a surrogate endpoint. Second, the effect of lifestyle modification on BP and CV outcomes may vary depending on sex, age, and ethnicity.13–16 Few studies of lifestyle intervention have incorporated sufficient numbers of older adults or minority populations to provide strong evidence for specific recommendations for these important groups.
Dietary Interventions for Blood Pressure Control
Traditional approaches to the study of diet and BP have focused on individual nutrients. As considered in depth in Chapter 46, a more recent concept recognizes that consumption of specific nutrients occurs in the context of food in a diet. Hence the contemporary approach to studies of nutrition and health focuses more on dietary patterns rather than on specific nutrients. This section first considers dietary patterns that have undergone evaluation with respect to BP control, followed by individual macronutrients and micronutrients of particular interest in this regard.
Two dietary patterns in particular have undergone contemporary and rigorous study in relation to BP control: the Mediterranean diet pattern and the Dietary Approaches to Stop Hypertension (DASH) diet pattern. Table 44-1 provides brief definitions of the Mediterranean and DASH diet patterns derived from the 2013 American Heart Association/American College of Cardiology (AHA/ACC) guideline on lifestyle management to reduce CV risk.17 (See Chapter 46 and references 18 and 19 for further detail.)
The Mediterranean Diet Pattern
The recent publication of the PREDIMED (Prevención con Dieta Mediterránea) study stimulated interest among CV specialists in the potential benefits of a Mediterranean diet.20 This trial showed an overall benefit in CV outcomes in the dietary intervention groups driven by a decrease in stroke, an endpoint closely associated with BP. The comparator group consumed a low-fat diet. BP data are not yet available from this study, yet at baseline more than 80% of the participants had hypertension, defined as systolic blood pressure (SBP) higher than 140 mm Hg, diastolic blood pressure (DBP) of 90 mm Hg or higher, or the use of antihypertensive therapy. Consumption of a Mediterranean diet pattern correlated with improvement in numerous biomarkers associated with CV benefit—ranging from reductions in BP21 to anti-inflammatory effects, as assessed by reduced C-reactive protein levels.22 Yet the most recent AHA/ACC guidelines on lifestyle management assessed the strength of evidence as being low regarding consumption of a Mediterranean diet pattern versus a low-fat dietary pattern despite reductions in SBP ranging from 2 to 7 mm Hg following an intervention.
The DASH Diet Pattern
The DASH diet (Table 44-1) evolved from studies supported by the U.S. National Heart, Lung and Blood Institute (NHLBI). These randomized, controlled DASH feeding studies showed that this dietary pattern could lower SBP by more than 5 mm Hg in adults with moderate hypertension when compared with the control diet.23 The effect size was larger in members of minority groups than in white subjects participating in the studies. A follow-up study known as DASH–Sodium tested the hypothesis that salt restriction in addition to the DASH diet would further reduce BP by evaluating three different levels of sodium content (3, 2.4, or 1.5 g daily). Over the 30 days of intervention, the low-salt diet produced a drop in SBP of almost 9 mm Hg.24 The 2013 AHA/ACC guidelines consider the strength of evidence high for adherence to the DASH diet in individuals with hypertension.17
Sodium Consumption and Blood Pressure
The relationship between sodium and BP provides a particularly important example of the necessity of considering public health interventions, as well as lifestyle change, in individual patients to control CV risk. The effects of sodium intake on BP and the CV benefits of limiting sodium consumption have proved contentious and controversial over many decades. In May 2013, the U.S. Institute of Medicine (IOM) released a report on sodium intake in populations in which the evidence in this regard was assessed.25 The report particularly addressed the concern that more stringent dietary restriction of sodium might be associated with increased overall health risk. The IOM committee identified many methodologic concerns about the evidence base regarding sodium intake and health, yet the report concluded that the weight of the evidence supported a link between higher levels of sodium consumption and CV risk but judged the evidence insufficient to support a restriction in sodium intake to below 2.3 g daily. Of particular interest to cardiologists, this evidentiary IOM review suggested that low sodium might worsen outcomes in individuals with severe heart failure. Ultimately, the IOM panel did not interpret the current evidence base as supportive of efforts to lower dietary sodium to 1.5 g daily for the general public. They called for further investigation to probe the health effects of salt intake in the range of 1.5 to 2.3 g daily.
The 2013 AHA/ACC lifestyle management guidelines concluded that in adults 25 to 80 years of age with an SBP of 120 to 159 mm Hg, reducing sodium intake lowers BP.17 They further found the evidence strong that for adults 30 to 80 years of age with or without hypertension, reduction of sodium intake by approximately 1 g daily lowers SBP by 3 to 4 mm Hg. Despite specific concerns, they judged the strength of evidence insufficient to support an association between sodium intake and the development of heart failure or that it could influence CV outcomes in patients with established heart failure.
Potassium Intake and Blood Pressure
Considerable observational data suggest an association between high potassium intake and lower BP. Increased consumption of potassium may lower BP, particularly in blacks as compared with whites. Even though the American Society of Hypertension (ASH) recommends an increase in potassium intake to 4.7 g daily (the level provided in the DASH diet),13 the 2013 AHA/ACC lifestyle guidelines find the strength of evidence insufficient to establish a relationship between increased dietary potassium and lower BP or altered risk for coronary heart disease, heart failure, or CV mortality.17
Carbohydrate Consumption and Blood Pressure
The observational data base yields disparate data regarding the effect of the amount and composition of dietary carbohydrates on BP. OmniHeart (Optimal Macronutrient Intake Trial to Prevent Heart Disease) showed that exchanging dietary carbohydrate for either protein or monounsaturated fat lowers BP. This study included 164 subjects with an SBP at baseline of 120 to 159 mm Hg.26 Albeit small, this well-designed and well-conducted study showed not only a decrease in BP but also a concomitant improvement in lipid profile. The 2013 AHA/ACC lifestyle guidelines considered the strength of evidence insufficient to make recommendations regarding the potential benefits of low-glycemic diets versus high-glycemic diets for individuals without diabetes.17
Ethanol Intake and Blood Pressure
A large body of observational evidence supports higher levels of BP in association with greater alcohol intake. A meta-analysis of self-reported decreases in alcohol intake showed that it lowered SBP by more than 3 mm Hg and DBP by more than 2 mm Hg.27 On the basis of the observational data and this meta-analysis, the ASH recommends limiting consumption to one alcoholic drink per day in women and no more than two alcoholic drinks per day in men.
Sugar-Sweetened Beverages
The increased consumption of sugar-sweetened beverages (SSBs) worldwide has been linked to the epidemic of obesity, particular in the young.28,29 Evidence also supports an association between increased consumption of SSBs and higher levels of BP. A prospective analysis of the PREMIER study showed that after adjustment for confounders, a reduction in SSBs by one serving daily resulted in an almost 2–mm Hg decrease in SBP.30 An international study of the effect of macronutrients and micronutrients on BP reported cross-sectional associations of SSBs with BP and found that one serving of an SSB daily was associated with a difference in SBP of greater than 1.5 mm Hg. This analysis showed a direct relationship between fructose and glucose intake with BP.31 These observational and trial data suggest that curbing SSB consumption could lower BP in the population and that restriction of SSB intake should be considered in individuals with established hypertension.
Other Macronutrients and Micronutrients and Blood Pressure Control
Many studies have linked other macronutrients and micronutrients with BP control. The foregoing discussion has considered those supported by the strongest evidence base. Table 44-2 provides a more ample list of the dietary factors and dietary patterns implicated in BP control, with estimates of the strength of the evidence adapted from the ASH position paper on dietary approaches to lower BP.13
TABLE 44-2
Effects of Dietary Factors and Dietary Patterns on Blood Pressure: Summary of the Evidence
| HYPOTHESIZED EFFECT | EVIDENCE | |
| Weight | Direct | +/+ |
| Sodium chloride (salt) | Direct | +/+ |
| Potassium | Inverse | +/+ |
| Magnesium | Inverse | +/− |
| Calcium | Inverse | +/− |
| Alcohol | Direct | +/+ |
| Fat | ||
| Saturated | Direct | +/− |
| Omega-3 polyunsaturated | Inverse | +/+ |
| Omega-6 polyunsaturated | Inverse | +/− |
| Monounsaturated | Inverse | + |
| Protein | ||
| Total | Uncertain | + |
| Vegetable | Inverse | + |
| Animal | Uncertain | +/− |
| Carbohydrate | Direct | + |
| Fiber | Inverse | + |
| Cholesterol | Direct | +/− |
| Dietary patterns | ||
| Vegetarian diets | Inverse | +/+ |
| DASH-type dietary patterns | Inverse | +/+ |
Key to evidence: +/− = limited or equivocal evidence. +/+ = persuasive evidence, typically from clinical trials.
Modified from Appel LJ: ASH position paper: Dietary approaches to lower BP. J Am Soc Hypertens 3:321, 2009.
Obesity/Body Weight
Considerable observational data support a relationship between body mass index (BMI) and the development of hypertension. Overall, adiposity was strongly associated with incident hypertension in both blacks and whites in the NHANES (National Health and Nutrition Evaluation Survey) data.32 Visceral adiposity and other ectopic fat deposits may also be associated with hypertension. As with other components of “metabolic syndrome,” hypertension may develop in Asians at a lower waist circumference than in whites or blacks. In the Nurses’ Health Study, which monitored more than 80,000 women for 14 years, BMI correlated most strongly with incident hypertension among the six risk factors evaluated—with a hazard ratio of 4.7 for obese women versus those with a BMI of less than 23 kg/m2. The population attributable risk for the development of hypertension with a BMI higher than 25 kg/m2 was 50% (95% confidence interval, 49% to 52%). These data suggest that obesity constitutes a major risk for hypertension and that control of body weight might eliminate a great amount of the morbidity associated with hypertension and avoid pharmacotherapy with its attendant unwanted effects (Table 44-3).33
TABLE 44-3
Risk for Hypertension According to Individual Factors Evaluated on the Basis of Estimated Population Attributed Risk
| FACTOR | POPULATION ATTRIBUTED RISK (95% CONFIDENCE INTERVAL) |
| BMI ≥ 25 kg/m2 | 50% (49-52%) |
| Non-narcotic analgesic use | 17% (15-19%) |
| No DASH diet | 14% (10-17%) |
| No vigorous exercise | 14% (10-19%) |
| No or excessive alcohol | 10% (8-12%) |
| Folic acid use ≤ 400 µg/day | 4% (1-7%) |
Modified from Liebson PR: Diet, lifestyle, and hypertension and Mediterranean diet and risk of dementia. Prev Cardiol 2010;13:94, 2010.
Physical Activity
Epidemiologic and observational studies have linked insufficient physical activity to increased CV risk. Because physical activity influences both CV fitness and body weight and visceral adiposity, the mechanisms through which exercise interacts with CV risk factors—and potentially with outcomes—remain difficult to define. Moreover, the effects of physical activity depend on whether the activity involves aerobic exercise, strength training, or a combination of both. In the case of BP control, the response to physical activity may be heterogeneous. Some individuals may have increases in BP when they undergo exercise training, whereas others may have reductions. The effects of physical activity on BP also depend on whether acute effects during or immediately following exercise are measured versus chronic changes in this risk factor.34 An occasional hypertensive patient may even experience symptomatic hypotension immediately after exercise, thereby requiring a reduction in the dose of BP medication. As in other aspects of lifestyle intervention, few studies have examined actual CV outcomes rather than biomarkers of surrogate endpoints. A recent meta-epidemiologic analysis that included 4 exercise meta-analyses and 12 drug meta-analyses, including more than 300 randomized, controlled trials involving more than 300,000 participants, found that exercise interventions and some drug interventions provided similar mortality benefits.35 Some evidence supports a genetic basis in determining the BP response to exercise, but no clinically applicable findings have emerged from such genomic analyses thus far.36 Some evidence supports a decrease in biomarkers of inflammation with interval exercise training in patients with hypertension.37
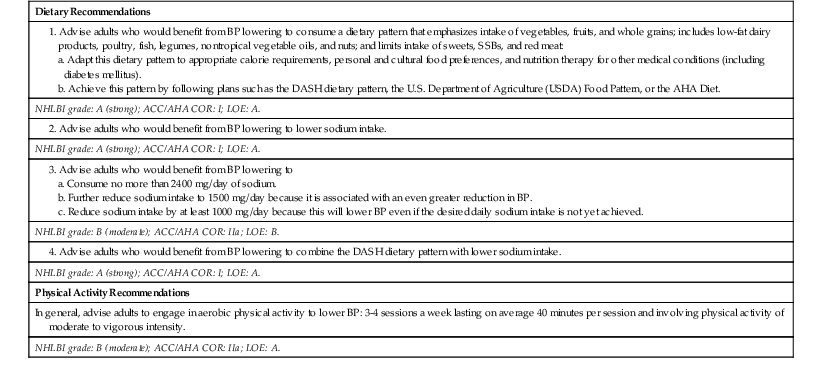
The 2013 AHA/ACC guideline summarizes an extensive evidentiary review that includes the 2008 report of the Physical Activity Guidelines Advisory Committee of the U.S. Department of Health and Human Services.38 The 2013 guideline database included 15 recent meta-analyses. The guideline states that in adults with or without hypertension, aerobic physical activity reduces SBP up to 5 mm Hg, with high strength of evidence. The committee concluded that the evidence was insufficient to provide an assessment of the effect of resistance exercise training on BP. They similarly pointed to a paucity of data regarding combined aerobic and resistance exercise intervention on regulation of BP. The committee provided a grade B recommendation that all adults engage in regular physical activity (Table 44-4).
TABLE 44-4
Diet and Physical Activity Recommendations for Lowering Blood Pressure
| Dietary Recommendations |
| NHLBI grade: A (strong); ACC/AHA COR: I; LOE: A. |
| NHLBI grade: A (strong); ACC/AHA COR: I; LOE: A. |
| NHLBI grade: B (moderate); ACC/AHA COR: IIa; LOE: B. |
| NHLBI grade: A (strong); ACC/AHA COR: I; LOE: A. |
| Physical Activity Recommendations |
| In general, advise adults to engage in aerobic physical activity to lower BP: 3-4 sessions a week lasting on average 40 minutes per session and involving physical activity of moderate to vigorous intensity. |
| NHLBI grade: B (moderate); ACC/AHA COR: IIa; LOE: A. |
COR = class of recommendation; LOE = level of evidence.
Modified from the Eckel RH, Jakicic JM, Ard JD, et al: 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Clin Cardiol 2013 Nov 7. pii: S0735-1097(13)06029-4. doi: 10.1016/j.jacc.2013.11.003. [Epub ahead of print.]
Cigarette Smoking
The effect of cigarette smoking on hypertension and outcomes in hypertensive patients remains difficult to define because of confounding by increases in waist girth with smoking cessation.39 Each cigarette evokes a transient pressor response that dissipates over the next hour. Despite the lack of precise mechanistic information regarding smoking and BP control, the overwhelming deleterious effect of smoking on CV risk, as well as the public health benefits of preventing the start of smoking and promoting cessation of smoking, renders this issue moot for public health and individual patient management.
Barriers to Adoption and Maintenance of Lifestyle Change and Possible Solutions
In practice, encouraging sustainable lifestyle change has proved extremely difficult. Substantial recent efforts have explored strategies and tools for encouraging the adoption of healthier lifestyles, including weight control, diet, and physical activity. Some challenges to lifestyle change identified in the literature will resonate with practitioners. Individuals express a low desire for, interest in, or awareness of dietary change, including weight loss, decreased sodium intake, smoking cessation, or reduced alcohol consumption. Barriers to adoption of physical activity recommendations include comorbid conditions that limit physical activity, as well as limited time.40 Contemporary adjuncts to the usual medical model for lifestyle intervention include Internet-based interventions, which are currently under intense evaluation.38,41–44 Given its critical importance for CV and metabolic health, effective measures for implementing and sustaining lifestyle change should remain an important goal for research and process improvement.
Antihypertensive Drugs
Although all hypertensive individuals should heed the lifestyle measure outlines above, most will also require drug therapy to optimize outcomes. Metaregression analyses of hundreds of thousands of hypertensive patients in randomized controlled trials (RCTs) have indicated that reduction in BP (hemodynamic load) explains most of the CV benefits of treating hypertension, with minor differences noted across major drug classes (Fig. 44-1).45 Reduced SBP confers the greatest magnitude of benefit in lowering the risk for stroke.
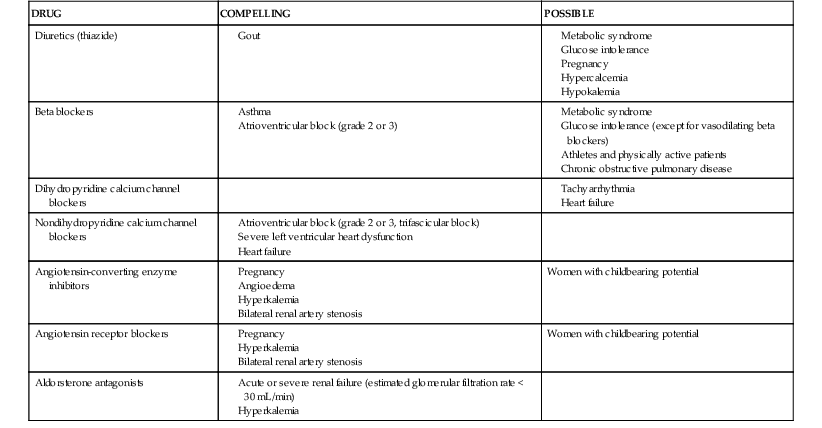
Oral antihypertensive drugs approved by the U.S. Food and Drug Administration (FDA) are shown in Table 44-5. Contraindications to specific drug classes are presented in Table 44-6. Preferred antihypertensive drug classes for specific patient subsets are listed in Table 44-7.
TABLE 44-5
Oral Antihypertensive Drugs
| DRUG | DOSE RANGE, TOTAL mg/day (DOSES PER DAY) |
| Diuretics | |
| Thiazide and Thiazide-Type Diuretics | |
| Chlorthalidone | 6.25-50 (1) |
| HCTZ | 6.25-50 (1) |
| Indapamide | 1.25-5 (1) |
| Metolazone | 2.5-5 (1) |
| Loop Diuretics | |
| Furosemide | 20-160 (2) |
| Torsemide | 2.5-0 (1-2) |
| Bumetanide | 0.5-2 (2) |
| Ethacrynic acid | 25-100 (2) |
| Potassium-Sparing Diuretics | |
| Amiloride | 5-20 (1) |
| Triamterene | 25-100 (1) |
| Spironolactone | 12.5-400 (1-2) |
| Eplerenone | 25-100 (1-2) |
| Beta Blockers | |
| Standard Beta Blockers | |
| Acebutolol | 200-800 (2) |
| Atenolol | 25-100 (1) |
| Betaxolol | 5-20 (1) |
| Bisoprolol | 2.5-20 (1) |
| Carteolol | 2.5-10 (1) |
| Metoprolol | 50-450 (2) |
| Metoprolol XL | 50-200 (1-2) |
| Nadolol | 20-320 (1) |
| Penbutolol | 10-80 (1) |
| Pindolol | 10-60 (2) |
| Propranolol | 40-180 (2) |
| Propranolol LA | 60-180 (1-2) |
| Timolol | 20-60 (2) |
| Vasodilating Beta Blockers | |
| Carvedilol | 6.25-50 (2) |
| Carvedilol CR | 10-40 (1) |
| Nebivolol | 5-40 (1) |
| Labetalol | 200-2400 (2) |
| Calcium Channel Blockers | |
| Dihydropyridines | |
| Amlodipine | 2.5-10 (1) |
| Felodipine | 2.5-20 (1-2) |
| Isradipine CR | 2.5-20 (2) |
| Nicardipine SR | 30-120 (2) |
| Nifedipine XL | 30-120 (1) |
| Nisoldipine | 10-40 (1-2) |
| Nondihydropyridines | |
| Diltiazem CD | 120-540 (1) |
| Verapamil HS | 120-480 (1) |
| Angiotensin-Converting Enzyme Inhibitors | |
| Benazepril | 10-80 (1-2) |
| Captopril | 25-150 (2) |
| Enalapril | 2.5-40 (2) |
| Fosinopril | 10-80 (1-2) |
| Lisinopril | 5-80 (1-2) |
| Moexipril | 7.5-30 (1) |
| Perindopril | 4-16 (1) |
| Quinapril | 5-80 (1-2) |
| Ramipril | 2.5-20 (1) |
| Trandolapril | 1-8 (1) |
| Angiotensin Receptor Blockers | |
| Candesartan | 8-32 (1) |
| Eprosartan | 400-800 (1-2) |
| Irbesartan | 150-300 (1) |
| Losartan | 25-100 (2) |
| Olmesartan | 5-40 (1) |
| Telmisartan | 20-80 (1) |
| Valsartan | 80-320 (1-2) |
| Direct Renin Inhibitor | |
| Aliskiren | 75-300 (1) |
| Alpha Blockers | |
| Doxazosin | 1-16 (1) |
| Prazosin | 1-40 (2-3) |
| Terazosin | 1-20 (1) |
| Phenoxybenzamine | 20-120 (2) for pheochromocytoma |
| Central Sympatholytics | |
| Clonidine | 0.2-1.2 (2-3) |
| Clonidine patch | 0.1-0.6 (weekly) |
| Guanabenz | 2-32 (2) |
| Guanfacine | 1-3 (1) (qhs) |
| Methyldopa | 250-1000 (2) |
| Reserpine | 0.05-0.25 (1) |
| Direct Vasodilators | |
| Hydralazine | 10-200 (2) |
| Minoxidil | 2.5-100 (1) |
| Fixed-Dose Combinations | |
| Aliskiren/HCTZ | 75-300/12.5-25 (1) |
| Amiloride/HCTZ | 5/50 (1) |
| Amlodipine/benazepril | 2.5-5/10-20 (1) |
| Amlodipine/valsartan | 5-10/160-320 (1) |
| Amlodipine/olmesartan | 5-10/20-40 (1) |
| Atenolol/chlorthalidone | 50-100/25 (1) |
| Benazepril/HCTZ | 5-20/6.25-25 (1) |
| Bisoprolol/HCTZ | 2.5-10/6.25 (1) |
| Candesartan/HCTZ | 16-32/12.5-25 (1) |
| Enalapril/HCTZ | 5-10/25 (1-2) |
| Eprosartan/HCTZ | 600/12.5-25 (1) |
| Fosinopril/HCTZ | 10-20/12.5 (1) |
| Irbesartan/HCTZ | 15-30/12.5-25 (1) |
| Losartan/HCTZ | 50-100/12.5-25 (1) |
| Olmesartan/amlodipine | 20-40/5-10 (1) |
| Olmesartan/HCTZ | 20-40/12.5-25 (1) |
| Olmesartan/amlodipine/HCTZ | 20-40/5-10/12.5-25 (1) |
| Spironolactone/HCTZ | 25/25 (1/2-1) |
| Telmisartan/HCTZ | 40-80/12.5-25 (1) |
| Trandolapril/verapamil | 2-4/180-240 (1) |
| Triamterene/HCTZ | 37.5/25 (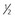 -1) -1) |
| Valsartan/HCTZ | 80-160/12.5-25 (1) |
| Valsartan/amlodipine/HCTZ | 80-160/5-10/12.5-25 (1) |
TABLE 44-7
Preferred Antihypertensive Drugs for Specific Conditions
| CONDITION | DRUG OR DRUGS |
| Patients with prehypertension | ARB? |
| Hypertensive patients in general | CCB, ACEI or ARB, D |
| Hypertension in older patients | CCB, ACEI or ARB, D |
| Hypertension with LVH | ARB, D, CCB |
| Hypertension in patients with diabetes mellitus | CCB, ACEI or ARB, D |
| Hypertension in patients with diabetic neuropathy | ARB, D |
| Hypertension in patients with nondiabetic chronic kidney disease | ACEI, BB, D |
| BP reduction for secondary prevention of coronary events | ACEI, CCB, BB, D |
| BP reduction for secondary prevention of stroke | ACEI + D, CCB |
| BP for patients with heart failure | D, BB, ACEI, ARB, aldosterone antagonists |
| Pregnancy | Methyldopa, BB, CCB |
| Aortic aneurysm | BB |
| Atrial fibrillation, ventricular rate control | BB, nondihydropyridine CCB |
BB = beta blocker; D = diuretic; LVH = left ventricular hypertrophy.
Modified from Mancia G, Fagard R, Narkiewicz K, et al: 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 31:1281, 2013.
First-Line Drug Classes
Most new practice guidelines3–12 (see Guidelines following this chapter) recommend initiating treatment of hypertension with one or more of the following three classes of first-line BP-lowering agents: (1) calcium channel blockers (CCBs); (2) renin-angiotensin system (RAS) inhibitors, either angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs); and (3) thiazide-type diuretics. Many RCTs and meta-analyses have shown that these drugs reduce the risk for nonfatal and fatal CV events. They have additive or synergistic effects when used in combination. Although beta-adrenergic blockers are first-line drugs for angina and heart failure, experts disagree whether they should be included among the first-line drugs for uncomplicated hypertension because of their inferior stroke protection and increased risk for incident diabetes. Experts differ in the emphasis placed on thiazide-type diuretics.
Calcium Channel Blockers for Hypertension
CCBs are very popular antihypertensive drugs. They are generally well tolerated, do not require monitoring with blood tests, and have proved safe and effective in many large RCTs. CCBs also have antianginal and some antiarrhythmic effects and seem to provide more protection against stroke than other antihypertensive agents do. More recent data have allayed concerns raised in the mid-1990s that CCBs cause excess coronary events. For example, ALLHAT (Antihypertensive Lowering to Prevent Heart Attack Trial) and subsequent RCTs showed that CCBs (represented by amlodipine) prevent coronary events as effectively as diuretics and RAS blockers do.46
Mechanism of Action
All CCBs block the opening of voltage-gated (L-type) Ca2+ channels in cardiac myocytes and vascular smooth muscle cells. They lower BP by causing peripheral arterial dilation, with the rank order of potency being dihydropyridines > diltiazem > verapamil.
Clinical Use
Amlodipine, by far the best studied of the dihydropyridine CCBs, has undergone evaluation in multiple RCTs. In ALLHAT, amlodipine was equivalent to chlorthalidone (a potent thiazide-like diuretic) and lisinopril (an ACEI) in protecting against nonfatal coronary events, stroke, and death but provided less protection against heart failure.46 Advantages of amlodipine include predictable dose-dependent potency, once-daily dosing because of its long half-life, tolerability, and cost. Some retail drug stores offer generic amlodipine for $10 per month. Unlike diuretics and RAS inhibitors, a high-salt diet or concurrent nonsteroidal anti-inflammatory drug (NSAID) therapy does not compromise the effectiveness of dihydropyridine CCBs. These drugs have some diuretic action (because of dilation of the afferent renal arteriole), which may reduce requirements for additional diuretic therapy in patients with mild hypertension. Unlike ACEIs, they are equally potent in lowering BP and preventing hypertensive complications in black and nonblack patients.46 ASCOT (Anglo-Scandinavian Cardiovascular Outcomes Trial)47 and the ACCOMPLISH (Avoiding Cardiovascular Events Through Combination Therapy in Patients Living with Systolic Hypertension)48 trial indicated that amlodipine plus an ACEI is one of the most effective drug combinations for preventing CV complications of hypertension. For comparable reductions in office (and ambulatory) BP, amlodipine/ACEI combination therapy improved CV outcomes better than did beta blocker/thiazide combination therapy in ASCOT or than did ACEI/thiazide combination therapy in ACCOMPLISH. Multiple fixed-dose single-pill combinations of amlodipine with an ACEI or an ARB have become available; some have added a thiazide for triple therapy.
Dihydropyridine CCBs such as amlodipine are less renoprotective than ACEIs or ARBs in patients with proteinuric chronic kidney disease (CKD); such patients should not receive amlodipine as first-line therapy, but a CCB may be useful as adjunctive therapy after initiation of appropriate first-line therapy with an ACEI or ARB and a diuretic. Verapamil is weakly antihypertensive and has limited usefulness because of dose-dependent constipation. Diltiazem is intermediate in potency between verapamil and the dihydropyridines and is usually well tolerated.
Side Effects
The principal side effect of the dihydropyridines is dose-dependent ankle edema. With amlodipine, ankle edema is far more common with a 10-mg dose than with 2.5- or 5-mg doses. This edema appears to be vasogenic because of selective arterial dilation and can respond to concomitant therapy with an ACEI or ARB that causes balanced arterial and venous dilation. Long-acting dihydropyridine CCBs are rarely associated with flushing and headache. All CCBs can cause gingival hyperplasia, a rare side effect that is reversible if detected early but can lead to several dental problems if the CCB therapy is not suspected as the cause. Verapamil and diltiazem can impair cardiac conduction, especially in older patients also receiving digoxin, beta blockers, or central sympatholytic agents.
Renin-Angiotensin Inhibitors for Hypertension: Angiotensin-Converting Enzyme Inhibitors, Angiotensin Receptor Blockers, and Direct Renin Inhibitors
RAS inhibitors are among the best tolerated of the antihypertensive drugs. The recent large study ONTARGET (Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial) showed comparable effects of the ACEI ramipril and the ARB telmisartan with regard to reducing CV events and preventing deterioration of renal function in high-risk hypertensive patients.49 Other data suggest that ARBs may provide slightly more protection against stroke. However, in general, the outcomes of many RCTs have not substantiated the hypothesis that RAS inhibitors produce important BP-independent benefits in hypertensive patients. The direct renin inhibitor (DRI) aliskiren is one of the newest BP drugs, but there are no completed or ongoing RCTs of aliskiren monotherapy. “Dual RAS blockade”—either with an ACEI plus an ARB or with aliskiren plus an ACEI or ARB—is now contraindicated. These combinations must be avoided because they produce more hypotension, accelerate the decline in renal function, and cause more hyperkalemia (see below).
Mechanisms of Action
ACEIs block conversion of the inactive precursor angiotensin I (A I) to A II. ARBs block the action of A II on the type 1 angiotensin receptor. The DRI aliskiren blocks the conversion of prorenin to renin, thereby blocking RAS activation at its origin. High levels of circulating prorenin may stimulate A I receptor–independent signaling pathways, which are both potentially beneficial and potentially harmful.
Clinical Use
ACEIs are easy to use and have a rather flat dose-response curve. In ALLHAT, ACEI monotherapy with lisinopril was equivalent to amlodipine or chlorthalidone monotherapy in all aspects except for producing a smaller reduction in BP and thus less stroke protection in black hypertensive individuals.46 As monotherapy, ACEIs are generally less effective in lowering BP in black patients and in older patients with low-renin hypertension, but they are quite effective in these groups when combined with a low-dose diuretic or CCB. In meta-analyses, ACEIs have been shown to be equivalent to CCBs in protecting against coronary events, slightly less effective in protecting against stroke, but better in protecting against heart failure.50
ARBs may confer the same benefits as ACEIs in treating hypertension while avoiding the ACEI-related cough (see below). Current $4 per month formularies include generic ACEIs but no ARBs. Losartan is the first ARB to become generic.
ACEIs and ARBs have become standard first-line antihypertensive therapy for patients with diabetic and nondiabetic CKD, but evidence has shown that RAS inhibitors provide superior renal protection than other antihypertensive agents do only for proteinuric CKD,11 as in AASK (African American Study of Kidney Disease). Head-to-head comparison in the large study ONTARGET has indicated that ACEIs and ARBs have comparable effects on renal function.51 For hypertensive individuals with normal baseline renal function, ACEIs and ARBs have not demonstrated greater renoprotective effects than other classes of antihypertensive agents.52
Although animal studies and retrospective meta-analyses have suggested that ACEIs and ARBs may prevent or slow progression from glucose intolerance to type 2 diabetes, the trial evidence is weak.11 In meta-analysis, ARBs produce more regression of left ventricular hypertrophy (LVH) than do other antihypertensive drugs.53
Side Effects
All RAS inhibitors are contraindicated in pregnancy because they cause fetal renal agenesis and other birth defects. The most common side effect of ACEIs is a dry cough, which is more common in black patients and more common still in Asian patients. ACEIs block the degradation of bradykinin, which activates the nociceptive sensory fibers in the lungs that trigger the cough. Bradykinin may also underlie ACEI-induced angioedema, a much less common adverse effect. If a cough develops in a patient taking an ACEI who needs RAS blockade, an ARB should be substituted. Only isolated instances of cough or angioedema associated with ARBs have been reported. ACEIs and ARBs can provoke hyperkalemia in the setting of CKD or diabetes with type 4 renal tubular acidosis. In patients with stage 3 CKD with proteinuria, initiation of ACEI or ARB therapy is often associated with a small transient increase in serum creatinine; therapy can be continued unless the elevation in creatinine is greater than 30%, an indication to decrease the dose or temporarily withhold therapy.
ACEIs and ARBs have been used together for extra renal protection in proteinuric patients. Yet the results of ONTARGET showed that such dual RAS blockade increases serious renal outcomes, hypotensive events, and hyperkalemia when compared with monotherapy with either agent alone.51 The combination of an ACEI or ARB with aliskiren entails similar risks,54,55 which has caused the FDA to issue a black box warning and to halt marketing of the fixed-dose combination. Moreover, the COOPERATE (Combination Treatment of Angiotensin-II Receptor Blocker and Angiotensin-Converting-Enzyme Inhibitor in Non-diabetic Renal Disease) trial, which had provided the earlier evidence supporting the practice of dual RAS blockade, was retracted from publication in Lancet on the basis of scientific misconduct.56
Diuretics for Hypertension
Diuretics are among the oldest and most effective antihypertensive medications. Even though diuretics are inexpensive, generic forms of most other BP drug classes have also become inexpensive. Diuretics have been the cornerstone of antihypertensive therapy since the first Joint National Committee (JNC) report in 1977 through the 2003 JNC 7 report. The 2103 scientific advisory statement from the AHA, ACC, and Centers for Disease Control and Prevention (CDC)7 still recommends thiazide diuretics as the best choice to initiate antihypertensive therapy, whereas the 2014 report of the JNC 8 committee members9 and most other recent guidelines list them as one of three first-line choices (see Table 44G-2 in the Guidelines section). Multiple RCTs have shown that thiazide-type diuretics reduce coronary events, strokes, and heart failure in elderly patients.4 In ALLHAT, the diuretic was equally effective as the ACEI and CCB in preventing coronary events and strokes, more effective than the CCB in preventing heart failure, and in black patients, more effective than the ACEI in preventing strokes. When combined with most other classes of antihypertensive drugs, diuretics exert a synergistic effect on BP reduction, but in the more recent ACCOMPLISH trial, the combination of an ACEI with a CCB yielded better outcomes than did combination with hydrochlorothiazide (HCTZ).48 Despite the popularity of HCTZ in the United States, the bulk of clinical trials supporting the benefits of diuretic therapy for hypertension did not use HCTZ but rather chlorthalidone, a thiazide-like diuretic that is more potent and longer lasting than HCTZ (see below). Thiazide and thiazide-like diuretics (especially in higher doses) cause more metabolic side effects and more erectile dysfunction than do ACEIs or CCBs and have higher discontinuation rates.57
Mechanisms of Action
With initiation of diuretic therapy, contraction of blood volume causes the initial fall in BP. With continued therapy, blood volume is partially restored, and vasodilator mechanisms (e.g., opening of adenosine triphosphate [ATP]-sensitive K+ channels) sustain the antihypertensive action. Loop diuretics block Na+-K+-2Cl− transport in the thick ascending loop of Henle. Thiazide diuretics and thiazide-like diuretics (chlorthalidone, indapamide) block the Na+-Cl− cotransporter in the distal convoluted tubule. Spironolactone and eplerenone prevent aldosterone from activating the mineralocorticoid receptor, thereby inhibiting downstream activation of the epithelial sodium channel (ENaC), whereas triamterene and amiloride block ENaC directly; because less sodium is presented to the Na+,K+-ATPase on the vascular side of the collecting duct cells, less potassium is excreted in urine.
Clinical Use: Chlorthalidone rather than Hydrochlorothiazide
Even though HCTZ has enjoyed widespread use in clinical practice, chlorthalidone is the choice for practicing evidence-based medicine. Greater effectiveness of chlorthalidone than HCTZ is strongly suggested by post hoc analysis of the MRFIT (Multiple Risk Factor Intervention Trial) data, which showed better outcomes with chlorthalidone,58 by a network meta-analysis,59 and by a small single-center ambulatory BP monitoring study showing a much longer duration of action.60 A 25-mg dose of chlorthalidone is roughly equivalent in potency to a 50-mg dose of HCTZ. Loop diuretics are less effective BP-lowering agents and should be reserved for treating hypertension in the setting of advanced CKD (stage 3 or higher). Chlorthalidone may also be effective in patients with stage 3 CKD.
Diuretics enhance the potency of all other classes of antihypertensive agents. Thiazide and thiazide-like diuretics combine particularly well with ACEIs and ARBs, which blunt the reactive RAS activation and thus increase antihypertensive efficacy. Such low-dose combinations should also reduce dose-dependent diuretic side effects, but no formal dose-finding studies are available to clarify their use in clinical practice.
Side Effects
Thiazides and thiazide-like diuretics can aggravate glucose intolerance (particularly in higher doses and when used in combination with a beta blocker), cause hypokalemia and hypomagnesemia, precipitate gout, and elevate serum lipids with increased hepatic triglyceride content61; rarely, they cause photosensitive dermatitis. They are more likely than any other antihypertensive drugs to cause erectile dysfunction. These drugs are the most common cause of severe hyponatremia, especially in older adults.62,63 Although less well recognized than thiazide-induced hypokalemia, thiazide-induced hyponatremia is a common reason why some elderly hypertensive individuals simply cannot tolerate even low-dose thiazides. In hypertensive patients with CKD, high doses of loop diuretics may precipitate acute renal failure, especially if combined with high-dose ACEI or ARB therapy.
Add-On Drug Classes for Difficult Hypertension
Aldosterone Antagonists
Low-dose spironolactone (12.5 to 100 mg daily) is widely recommended as a highly effective add-on drug for difficult cases of hypertension.10,11,64 This recommendation is based on small single-site series and post hoc analysis of ASCOT, which used spironolactone (12.5 to 25 mg daily) as a fourth-line therapy. Eplerenone is a much more specific antagonist that avoids the infrequent sexual side effects of low-dose spironolactone (painful gynecomastia, erectile dysfunction, nonmenstrual uterine bleeding). Hyperkalemia must be avoided when using these agents in patients with kidney disease.
Beta-Adrenergic Blockers
Vasodilating beta blockers (labetalol, carvedilol, and nebivolol) are also highly effective add-on drugs for difficult hypertension; standard beta blockers (e.g., metoprolol, atenolol) are not.
Mechanism of Action
With the initiation of standard beta-blocking drug therapy, BP changes little at first because a compensatory increase in peripheral resistance offsets the fall in cardiac output. Over time, BP falls progressively as the peripheral vasculature relaxes. Thus the antihypertensive effect of beta blockade involves decreases in cardiac output (beta1 receptors), renin release (beta1 receptors), and norepinephrine release (prejunctional beta2 receptors). The prototype beta blocker propranolol nonselectively blocks both beta1 receptors and beta2 receptors. Other standard beta blockers (metoprolol, atenolol, acebutolol, and bisoprolol) are relatively cardioselective. In low doses they exert a greater inhibitory effect on beta1 receptors than on beta2 receptors, but selectivity is lost at high doses. Vasodilating beta blockers such as labetalol or carvedilol also block alpha-adrenergic receptors, whereas nebivolol stimulates endogenous production of nitric oxide.
Clinical Use and Side Effects
Standard beta blockers have rather weak BP-lowering action. Several RCTs and meta-analyses have indicated that standard beta blockers such as atenolol and metoprolol provide stroke protection inferior to that with ACEIs, ARBs, CCBs, or diuretics. They provide modest protection against CV events but do not reduce all-cause mortality.65 Standard beta blockers also increase the risk for diabetes, particularly when combined with a diuretic. Common side effects such as fatigability cause high discontinuation rates.57 Beta blockers can impair cardiac conduction and precipitate acute bronchospasm in adults who had asthma in childhood. All beta-blocking drugs promote weight gain. Vasodilating beta blockers are much more potent antihypertensive agents and do not adversely affect glucose tolerance, but they have not undergone evaluation in large RCTs.65 Data are also lacking on whether branded nebivolol is more cardioprotective than generic carvedilol, which is now included in $4 per month formularies. Labetalol is effective treatment of hypertensive urgency but is too short acting to be recommended for chronic hypertension management.
Alpha-Adrenergic Blockers
Mechanism of Action
By blocking the interaction of norepinephrine on vascular alpha-adrenergic receptors, these drugs cause peripheral vasodilation, thereby lowering BP. By increasing blood flow in skeletal muscle, alpha blockers increase insulin sensitivity. By dilating urethral smooth muscle, they improve symptoms of prostatism. Prazosin, doxazosin, terazosin, and intravenous phentolamine selectively block alpha1 adrenoceptors; phenoxybenzamine blocks both alpha1 and alpha2 receptors.
Clinical Use and Side Effects
Phenoxybenzamine remains the drug of choice for preoperative management of pheochromocytoma (see Chapter 81); after alpha blockade is achieved, a beta blocker should be added to block an otherwise excessive reflex tachycardia. Selective alpha1-blocking drugs are not first-line agents and should not be used as monotherapy because their propensity to cause fluid retention can lead to tachyphylaxis and unmask or exacerbate heart failure. When used in a combination regimen that includes a diuretic, however, they are effective add-on therapy for difficult hypertension and are particularly useful in older men with prostatism. Although marketed specifically for prostatism and not as an antihypertensive agent, the selective alpha1A– blocker tamsulosin lowers BP in some men.
Central Sympatholytics
Mechanism of Action
Stimulation of postsynaptic alpha2-adrenergic receptors and imidazoline receptors in the central nervous system lowers central sympathetic outflow, whereas stimulation of presynaptic alpha2 receptors causes feedback inhibition of norepinephrine release from peripheral sympathetic nerve terminals. These combined actions reduce adrenergic drive to the heart and peripheral circulation.
Clinical Use and Side Effects
The central sympatholytics are best reserved for short-term oral treatment of hypertensive urgency. They are potent antihypertensive agents that may be needed as add-on therapy for very difficult hypertension, but their troublesome central nervous system side effects reduce quality of life. To avoid rebound hypertension between doses, short-acting clonidine must be given every 6 to 8 hours or, whenever possible, discontinued via a gradual tapering schedule.66 Rebound hypertension is less of a problem with longer-acting preparations (e.g., guanfacine, clonidine patch). Alpha-methyldopa remains useful for the management of hypertension in pregnancy but is no longer a first-line therapy.
Direct Vasodilators
Mechanism of Action
Minoxidil and hydralazine are potent hyperpolarizing arterial vasodilators that work by opening vascular ATP-sensitive K+ channels.
Clinical Use
By causing selective and rapid arterial dilation, both drugs induce profound reflex sympathetic activation and tachycardia. Hydralazine is useful for the treatment of preeclampsia. A combination of hydralazine plus nitrates is useful for the treatment of heart failure, specifically in non-Hispanic black patients, in whom hypertensive heart disease causes heart failure most commonly (see Chapters 25 and 27). Severe hypertension accompanying advanced CKD is the main indication for minoxidil, which must be combined with a beta blocker to prevent excessive reflex tachycardia and with a loop diuretic to prevent excessive fluid retention. Institution of hemodialysis is usually a more effective means of controlling hypertension in this setting.
Percutaneous Interventions for Management of Blood Pressure
Renal Denervation (also see Chapter 43)
Percutaneous catheter-based radiofrequency ablation of the renal nerves (referred to as renal denervation [RDN]) has already entered clinical practice in Europe and Asia as a novel treatment of drug-resistant hypertension, with clinical guidelines being published in 2013.67 Based on impressive but unblinded data from phase I and phase II trials and office BP measurements, these guidelines will need to be reevaluated in light of a 2014 press release reporting that the blinded pivotal U.S. phase III trial (Symplicity HTN-3) did not reach its primary efficacy endpoint of a 15–mm Hg or greater reduction in office-based SBP in the RDN group versus the control group.68 Further research will be needed to conclusively determine the relative merits of RDN versus optimal medication management, patient subsets who would be most likely and least likely to benefit, sustainability of the therapeutic benefit in view of possible reinnervation, and long-term safety.69
Mechanism of Action (also See Chapter 43)
RDN therapy is derived from the premise that overactivity of the sympathetic nervous system contributes importantly to hypertension, especially severe drug-resistant hypertension. The targets of RDN are both the efferent postganglionic renal sympathetic nerves and the renal sensory (afferent) nerves. Overactivity of efferent renal nerves can cause renal vasoconstriction, stimulate renin release, and impair natriuresis (see Fig. 43-6). Overactivity of renal afferent nerves can trigger reflex efferent sympathetic nerve activation not only in the kidney but also in the heart (thereby promoting increased cardiac output, LVH, and atrial fibrillation), skeletal muscle (promoting increased vascular resistance and insulin resistance), and spleen (promoting T cell activation with secondary vascular inflammation and impaired endothelial function) (see Fig. 43-7).
Clinical Use
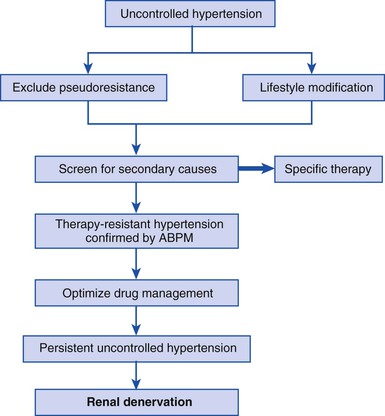
RDN is a percutaneous procedure with short recovery times and no significant systemic side effects (such as orthostatic hypotension or fatigue), the latter being a major advantage over central sympatholytic drugs. The Symplicity clinical trial program has demonstrated the short-term safety and efficacy of RDN for drug-resistant hypertension in phase I and II trials. Among patients with baseline SBP higher than 160 mm Hg despite treatment with an average of five antihypertensive drugs, substantial and progressively larger BP reductions were seen over 36 months of follow-up, with a goal SBP of lower than 140 mm Hg being achieved in 50% of patients.70 Based this evidence, the European Society of Cardiology recommended that “hypertensive patients are eligible for RDN if they have severe treatment-resistant hypertension defined by office SBP at least 160 mm Hg (≥150 mm Hg in type 2 diabetes) despite treatment with at least three antihypertensive drugs of different types in adequate doses, including one diuretic, which is equivalent to stage 2 or 3 hypertension.”67 Further recommendations for screening are shown in Figure 44-2 and recommendations for eligibility in Table 44-8. Again, these recommendations will need to be reassessed when data from the Symplicity HTN-3 trial are published.
TABLE 44-8
Eligibility Criteria Before Considering Renal Denervation