Fig. 6.1
Evaluation of pulmonary venous anatomy by TEE. In the center is a diagram of a person, facing front. The axis of 120° through the long axis of the left ventricle is drawn. When the person is viewed from the left side (left image), the anterior position of the left upper vein compared to the left lower vein is observed, as is the alignment of the 120° plane of imaging with both orifices. When the person is viewed from the right side (right image), the anterior position of the right upper vein compared to the right lower vein is observed, as is the alignment of the 60° plane of imaging with both orifices
Transesophageal Imaging
Imaging of Normal Systemic Veins
Identification of the atrial appendages can assist in differentiating the atria. The broad-based, blunt appearance of the right atrial appendage (RAA) is best appreciated in the sagittal plane (90°) in the mid esophageal bicaval (ME Bicaval) view, where the base of the RAA is seen anterior to the entrance of the SVC (Fig. 6.2a, Video 6.1). The narrow finger-like LAA can be seen at 0° with flexion or withdrawal of the probe from the mid esophageal four chamber view (ME 4 Ch), or alternatively it can be displayed by rotating the plane to 90° to obtain the mid esophageal two chamber view (ME 2 Ch) (Fig. 6.2b, Video 6.2) [7]. Within the RA, the crista terminalis may be seen as a linear structure extending from the RA wall close to the IVC to the septum adjacent to the SVC. This may be mistaken for the atrial septum. However, if this were the case, then the IVC would drain into the LA. This potential error is easily avoided simply by recalling that the IVC normally drains to the RA.
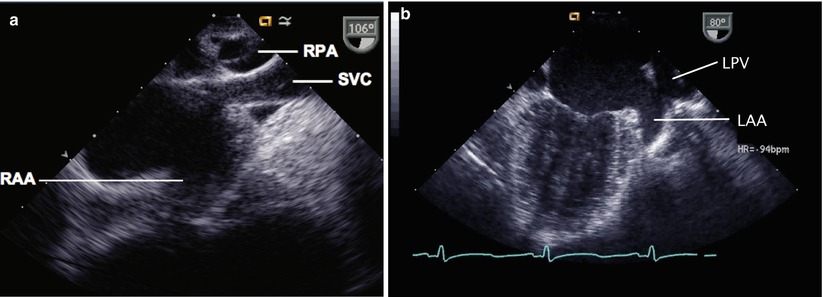

Fig. 6.2
(a) Transesophageal echocardiographic image of the right atrium viewed in the sagittal plane (mid esophageal bicaval view). The superior vena cava (SVC) is seen superiorly, and the right pulmonary artery (RPA) is seen in cross section as it courses behind the SVC. The broad-based right atrial appendage (RAA) is seen anteriorly. (b) Image of the left atrium in the mid esophageal two chamber view. The left upper pulmonary vein (LPV) is seen and directly anterior to this is the entrance of the narrow-based left atrial appendage (LAA) into the left atrial cavity
A normal RSVC can be imaged by starting in a horizontal plane (0°) at the ME 4 Ch view, then withdrawing the probe to the SVC-RA junction. The SVC will be bisected by the imaging plane. Once seen at 0°, if the multiplane angle is then rotated to 90° and the probe withdrawn, the SVC may be imaged in its long axis in the ME Bicaval view. The SVC course anterior to the RPA will be obvious in this plane. If the ascending aorta is seen, then clockwise rotation will result in imaging of the SVC in the normal heart.
The course of the IVC and HVs can be imaged using the ME Bicaval view with probe advancement past the level of the diaphragm or a lower esophageal situs short axis (LE Situs SAX) or deep transgastric approach at 0°. The course of these structures can be visualized by manipulating the probe and advancing and withdrawing the transducer within the esophagus to visualize which atrium the IVC is entering, as well as to identify the HVs draining into the IVC (Fig. 6.3, Video 6.3). One must remember to observe all the poles of the HVs, as a separate entrance from a posterior lobe can be easily missed. Normally the IVC enters the RA very midline, just adjacent to the atrial septum. The long axis of the IVC, and its entrance to the atrium, can be seen using the lower esophageal IVC long axis (LE IVC LAX) view, with the imaging plane at 90°, however the left-right orientation of the image is lost in this view (Fig. 6.4, Video 6.4). Keeping the same image position while adjusting the multiplane angle to 0°, while imaging the IVC, produces the LE Situs SAX view below the level of the heart. The TEE probe can be rotated in a clockwise-counterclockwise direction to determine the relation of the infradiaphragmatic vessels to one another and to the spine. The probe is then gradually withdrawn to demonstrate the presence of a venous (Eustachian) valve within the RA, the roof of the RA, and the connection of the RA to the SVC.
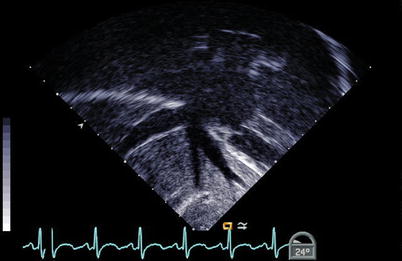
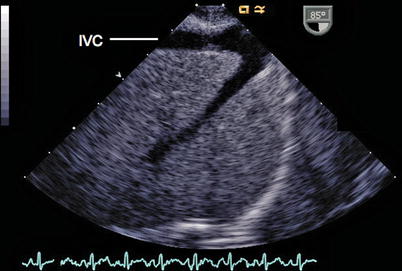

Fig. 6.3
The hepatic veins can be seen entering the right atrium in a nearly coronal plane from a deep transgastric window

Fig. 6.4
In this view the TEE probe is positioned in the lower esophageal inferior vena cava long axis window (probe angle adjusted to a 90° plane) to demonstrate the inferior vena cava (IVC). This view can be obtained by advancing the imaging probe from the mid esophageal bicaval view further into the esophagus. A hepatic vein is joining the IVC anteriorly and superiorly
The LIV can be imaged by starting from a ME 4 Ch view, rotating the transducer leftwards beyond the cardiac mass, towards the patient’s spine, until the descending aorta is seen in its short axis (Desc Ao SAX view). Keeping this in the center of the image sector, the transducer orientation is changed to about 90°–120° (descending aorta long axis or Desc Ao LAX view), and the probe withdrawn to image the proximal descending aorta. With further withdrawal and slight rightward probe shaft rotation, the transverse arch can be more proximally imaged in the upper esophageal aortic arch short axis (UE Ao Arch SAX) view. In this view the innominate vein is seen in cross section (short axis), anterior and superior to the aortic arch. Multiplane angle rotation to about 90 ° displays the upper esophageal aortic arch long axis (UE Ao Arch LAX) view where the innominate vein may also be seen crossing anterior to the aortic arch [8]. Although these views are possible, the tracheal air column may preclude a complete examination of the vascular structures at this level.
The coronary sinus (CS) is seen in the ME 4 Ch view by retroflexing the probe, where it traverses the atrioventricular groove behind the LA before entering the RA (Fig. 6.5, Video 6.5). To depict the relation between the CS and an atrial septal defect (ASD), an oblique (130°) mid esophageal view is useful.
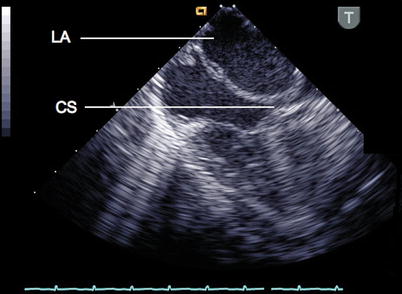

Fig. 6.5
In this mid esophageal four chamber view the probe has been retroflexed within the esophagus to demonstrate the coronary sinus (CS) and its relation to the left atrium (LA)
Imaging of Normal Pulmonary Veins
Several views can be used to evaluate the left and right pulmonary veins. Imaging begins with the transducer positioned behind the LA at 0°. From the ME 4 Ch view, the transducer is rotated leftwards (counterclockwise) to identify the left pulmonary veins, which usually course between the LAA and descending aorta (Fig. 6.6, Video 6.6). The LUPV enters the LA just lateral to the LAA from an anterior to posterior pathway. The LUPV is identified by slightly withdrawing and turning the probe to the left (from the LAA). The LLPV is then displayed by turning slightly farther to the left and advancing the probe into the esophagus. The LLPV enters the LA just below the LUPV. Alternatively, the exam can start from the ME 2 Ch view. Once the LAA is identified, slight probe withdrawal and turning to the left will demonstrate the LUPV above the LAA. After centering the LUPV on the display and rotating the multiplane angle to ~100–120°, the LLPV can be seen. Because the LLPV courses in a more lateral to medial direction, it is less suitable than the LUPV for Doppler interrogation of pulmonary venous blood flow [9].
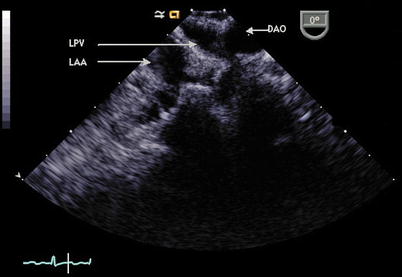

Fig. 6.6
In this image at 0°, the probe has been withdrawn and rotated leftwards (counterclockwise) from the mid esophageal four chamber view to demonstrate the relationship of the descending aorta (DAO), left upper pulmonary vein (LPV), and left atrial appendage (LAA) in a posterior to anterior position
The horizontal or transverse plane (0°) is less suited to imaging the right pulmonary veins, but often the right pulmonary veins can be viewed by first centering the RA in the image sector, then withdrawing the transducer until the RPA is encountered. The transducer is then rotated rightwards, and advanced slightly until RUPV flow is seen entering the LA from the right, and then the transducer advanced slightly further to image the RLPV (Fig. 6.7, Video 6.7).
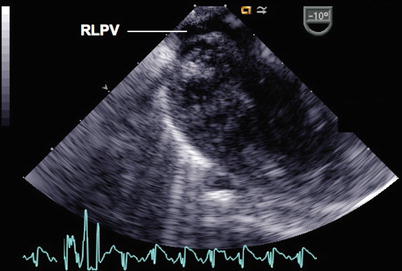

Fig. 6.7
In this image, the probe has been rotated rightwards (clockwise) in the mid esophageal four chamber view and advanced slightly within the esophagus to demonstrate the entrance of the right lower pulmonary vein (RLPV) into the left atrium
Another approach is to image the pulmonary veins on each side, in their long axis, as they course towards the LA [8]. One way to approach this is to recall that on each side, the upper veins attach to the LA more anteriorly than the lower veins. To view the left pulmonary veins using a multiplane transducer, the angle should be at about 120° (Fig. 6.8, Video 6.8). One can start from the mid esophageal aortic valve long axis (ME AV LAX) view of the left ventricle (LV) and left ventricular outflow tract and rotate leftwards, withdraw the probe slightly and use color Doppler to assist in locating the pulmonary veins by detecting the flow as these enter the LA. If the descending aorta is encountered, the transducer has been rotated too far leftwards (imaging more posteriorly than necessary), and must return slightly anterior by rotating clockwise. Often, one or the other left pulmonary veins may be visualized first, and the transducer beam orientation can be adjusted ±20°–30° until both veins can be seen lengthwise as they approach the LA. On occasion secondary tributaries are also seen, as this view seems to image the pulmonary veins along a greater length.
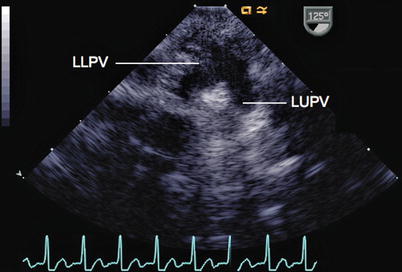

Fig. 6.8
View of the left pulmonary veins from a mid esophageal position (LLPV left lower pulmonary vein, LUPV left upper pulmonary vein) as they enter the left atrium, using an imaging plane of 125°. From the left atrium, the probe has been rotated leftwards to visualize the veins
For the right pulmonary veins, a similar approach may be taken, although at an almost perpendicular angle to the view for the left pulmonary veins. One can start at the mid esophageal right ventricular inflow-outflow (ME RV in-out) view, at about 30°–50°, and rotate rightwards (clockwise), to just past the SVC. Color Doppler may aid in initial identification and subsequent refinement of the imaging angle that will provide optimal views of the right pulmonary veins (Fig. 6.9, Video 6.9). When beginning to use this view, a helpful method of remembering which angle to use for the left and right pulmonary vein views is to recall that in the retrocardiac position, 120° provides imaging of the inflow and outflow of the LV, so it also provides imaging of the left veins when directed leftward and posteriorly. Similarly, in the retrocardiac position, 30°–50° provides imaging of the inflow and outflow of the right ventricle, and also provides imaging of the right veins when directed rightward and posteriorly.
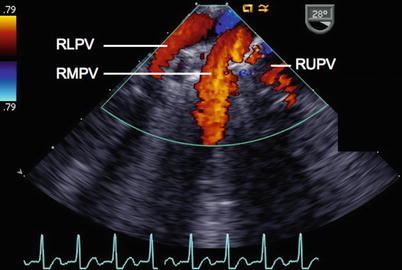

Fig. 6.9
View of the right pulmonary veins (RLPV right lower pulmonary vein, RMPV right middle pulmonary vein, RUPV right upper pulmonary vein) from a mid esophageal position, as they enter the left atrium. This view can be obtained with rightwards rotation of the probe, using an imaging plane of approximately 30–50°
Systemic Venous Abnormalities
Persistent Left Superior Vena Cava Draining to the Coronary Sinus
Over 90 % of cases of persistent left superior vena cava (LSVC) drain via a CS [10]. This condition is common, occurring in approximately 0.5 % of the general population [11–13] and in 3–10 % of patients with congenital heart defects [10, 14]. Although several reports cite total absence of the RSVC in 17 % of patients with a persistent LSVC [10, 14], this appears to be substantially less common in our experience.
Identification of an LSVC is important in a patient undergoing surgery, as it may alter the venous cannulation strategy. In addition, a LSVC to CS drainage pattern complicates atrial baffle procedures, because the CS is usually incorporated into the pulmonary venous atrium, which would not be wise given the increased volume of desaturated blood the CS would carry when an LSVC is connected to it [15].
The presence of bilateral SVCs has more significance in the execution of a bidirectional cavopulmonary anastomosis (BCPA). Some centers have reported increased risk for the bilateral BCPA in comparison to a single BCPA [16]. Central pulmonary artery (PA) hypoplasia may develop in this population after a bilateral BCPA, which may be ameliorated by the preservation of low grade antegrade pulmonary flow [17].
Bilateral SVCs may also impact heart transplantation, in that more LIV may need to be harvested with the donor heart, especially if the intended recipient has undergone a bilateral BCPA. In these patients, both the RSVC and LSVC can then be anastomosed to the donor LIV without need for additional reconstruction [15].
Other clinically important features of the presence of a LSVC may relate to the administration of retrograde cardioplegia and placement of catheters such as central venous lines and PA catheters as these may take an unusual course.
Transesophageal Echocardiography
A dilated CS may be identified as an echo-free region between the posterior mitral leaflet and the LA. To image the CS in its long axis, the examination is initiated in the ME 4 Ch view and the probe is slightly advanced within the esophagus (see Fig. 6.5). A short axis image of the CS can be seen anywhere from 90° to 120° in the ME 2 Ch and mid esophageal long axis (ME LAX) views (Fig. 6.10, Video 6.10). A dilated CS should initiate a search for a persistent LSVC. If this is not identified, other potential causes of a dilated CS should be investigated, such as anomalous pulmonary venous return or HVs draining to the CS, partial unroofing of the CS septum, CS orifice atresia or stenosis, elevated right atrial pressure, tricuspid regurgitation directed into the CS or coronary artery fistula to CS.
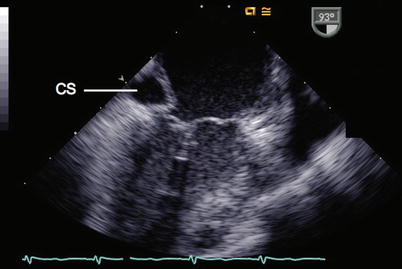

Fig. 6.10
In this mid esophageal two chamber view the left atrium and left ventricle are displayed. A dilated coronary sinus (CS) is demonstrated
The LSVC courses anterior to the left pulmonary artery (LPA), between the left pulmonary veins and the LAA. This vessel can be visualized lengthwise from mid to upper esophageal positions at about 60°–80° (Fig. 6.11, Video 6.11), descending to the left of the aortic arch and in front of the LPA towards the atrioventricular groove, where it continues into the CS. Pulsed and color Doppler confirm the direction of LSVC flow as being towards the heart. Vessels that resemble an LSVC, and have the same embryologic origin from the left cardinal vein system, but have venous flow away from the heart include a left vertical vein in patients with anomalous pulmonary venous drainage to the LIV, and a levoatrial cardinal vein in patients with mitral atresia and intact atrial septum [18, 19]. If the LSVC cannot be adequately imaged, injection of agitated saline into a left arm vein or left jugular vein, which results in opacification of the CS, will confirm the diagnosis [20, 21]. Sagittal views at 90° (ME Bicaval view) are useful for determining if an RSVC is present. When bilateral superior cavae are present, the LIV is usually either hypoplastic or absent [22–25]. When absence of the RSVC occurs, it is usually in the presence of an LSVC draining to a CS or directly to the LA [26–28]. In these cases, the LIV is well-developed.
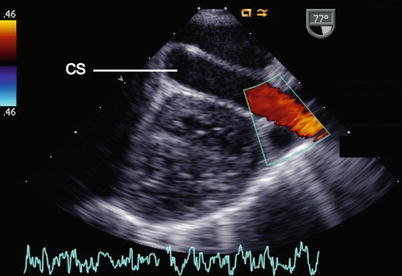

Fig. 6.11
View of the coronary sinus (CS) in its longitudinal plane. This receives a left superior vena cava as demonstrated by color flow mapping. This view can be obtained from initially obtaining the short axis view of the coronary sinus and rotating the transducer to 60°–80°
A dilated CS in association with a persistent LSVC has led to diagnostic errors as in some cases the opening of the CS in the RA has been misinterpreted for an ASD. This may be avoided by comprehensive anterior and posterior scanning from the ME 4 Ch view as the probe is anteflexed and retroflexed to display the anatomical details, color Doppler interrogation is applied, and structures of interest are examined in multiple planes.
Anomalous Subaortic Position of the Innominate Vein (Retroaortic Innominate Vein)
In approximately 1 % of patients with congenital heart disease (CHD), the LIV takes an anomalous course underneath the aortic arch [29–31]. This is seen more commonly in patients with tetralogy of Fallot or ventricular septal defect and pulmonary atresia. The incidence of a right aortic arch is higher in patients with this venous abnormality [29].
Its clinical significance lies in the potential for mistaking the LIV for a RPA, an error that may occur in the setting of branch PA hypoplasia [29]. The approach to venous cannulation during cardiac surgery may be affected by the presence of this anomaly.
Transesophageal Echocardiography
As the transesophageal windows may not allow for a comprehensive examination of the aortic arch and neighboring structures, this abnormality may not be easily assessed by TEE.
Persistent Left Superior Vena Cava Draining to the Left Atrium
In approximately 8 % of cases of a persistent LSVC, there is direct drainage of the LSVC into the roof of the LA. While this pattern may occur in right isomerism—a condition in which no CS is present [32], it is not diagnostic of this entity. Direct communication of the LSVC to the LA occurs rarely in isolation [33]. More commonly, this defect occurs in association with significant intracardiac anomalies such as ASD, common atrium, atrioventricular septal defect [14, 34–36], anomalous pulmonary venous return, coarctation of the aorta, tetralogy of Fallot, patent ductus arteriosus, transposition of the great arteries, and tricuspid atresia [35–37].
Transesophageal Echocardiography
The normal-sized CS is not easily visible on two-dimensional imaging. Due to the lack of dilation of the CS, this diagnosis can be missed unless the LSVC is visualized directly, or other very subtle clues are detected, such as a small or absent LIV and/or RSVC, which may lead to the search for an LSVC. Alternatively, if an LSVC has been noted (either by TEE or by the surgeon) but the CS is not dilated, one must have a strong suspicion that the LSVC drains directly into the LA. Contrast injection using agitated saline or albumin into a peripheral vein in the left arm or left head/neck vein will result in the appearance of “bubbles” in the LA.
Persistent LSVC Draining to a Coronary Sinus with a Coronary Sinus—Left Atrial Fenestration
This is similar to the entity described above (LSVC to CS), except a defect is present between the CS and the LA. This is also referred to as an unroofed CS. The CS orifice opens into the RA in its usual position. This condition may occur in isolation, or with associated heart malformations [38]. When atresia of one atrioventricular valve is present, the communication may represent the only egress of blood from the RA or LA in the absence of other communications at the atrial level [39–41]. Failure to recognize the defect in the CS septum may result in persistent interatrial shunting, and may cause postoperative cyanosis if the shunting is in the right to left direction.
Transesophageal Echocardiography
In the ME 4 Ch view, a dilated CS may present the first clue that an abnormality is present. The CS ostium may be dilated, and may be confused with a primum ASD—the mouth of the CS may be distinguished from a primum ASD on the basis of a normal-appearing mitral valve, and the CS orifice’s location posterior to the MV annulus. This is observed as a defect that becomes more visible when the probe is advanced from the usual mitral valve/atrial septal junction to the CS view. In the ME 2 Ch view, the dilated CS is seen, as is the septum separating the CS from the LA. A defect in this septum can be clearly demonstrated using this imaging plane (Fig. 6.12, Video 6.12).

Fig. 6.12
(a) In the mid esophageal two chamber view, the normal coronary sinus (CS) septum is seen. In this patient the coronary sinus is dilated. (b) The absence of septum is evident in this patient with a coronary sinus septal defect
Contrast echocardiography is helpful, as injection into a left arm vein will result in sequential opacification of the LSVC, CS, LA (Fig. 6.13), and finally the RA via the CS orifice [38]. This sequence may be variable as it depends on regional pressure gradients. For example, if the shunt direction is predominantly left to right (that is, from the LA to the CS via the defect, to the RA), injection into the left arm will stream predominantly into the RA, with negative contrast seen from the LA into the CS.
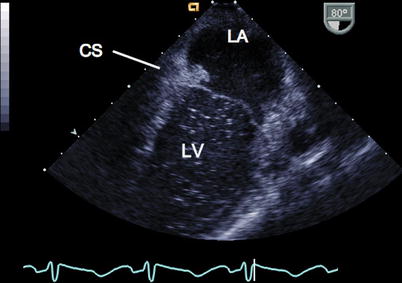

Fig. 6.13
In this image, which is the same orientation as demonstrated in Fig. 6.12, agitated saline was injected into the left arm. Opacification was seen first in the coronary sinus (CS), then a brief flash of contrast was seen entering the left atrium (LA), establishing the diagnosis of CS septal defect. This contrast was then washed back into the CS and into the right atrium, so that overall there was scant contrast seen in the left ventricle (LV) as shown
Right Superior Vena Cava Connection to Left Atrium
Drainage of a RSVC predominately or entirely into the LA is a rare finding, and has been the subject of several case reports [42–50]. In two-thirds of cases, patients presented with cyanosis. In every patient presenting with cyanosis there was no LSVC. The second most common finding leading to the diagnosis of RSVC to LA is a brain abscess.
Transesophageal Echocardiography
The course and location of the RSVC is relatively normal until its entrance to the atrium. Thus, imaging along the long axis of the SVC in the ME Bicaval view will often demonstrate the course of the RSVC, including its entrance to the LA, and the prominent right sinoatrial fold can often be seen in this view (Fig. 6.14, Video 6.13).
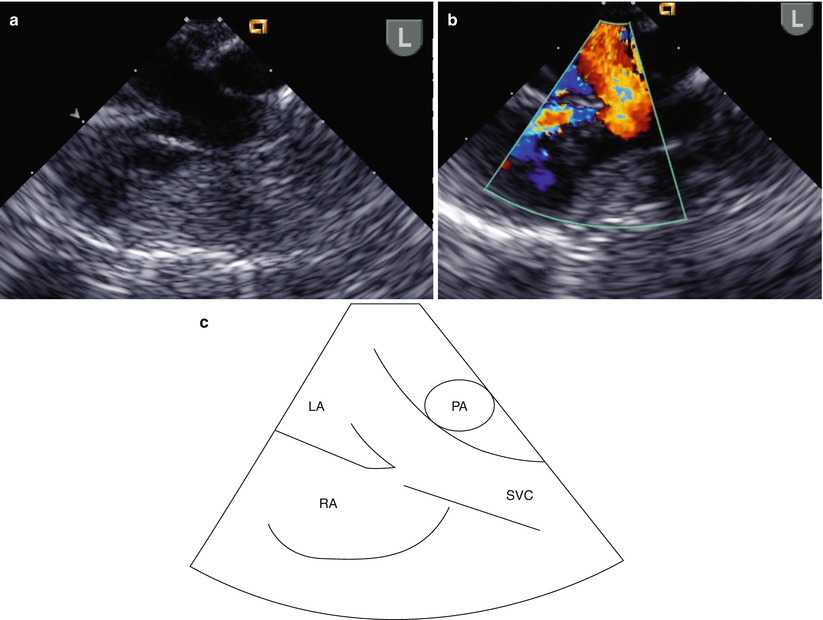

Fig. 6.14
This series of images in the longitudinal or sagittal plane in the mid esophageal bicaval view demonstrates the commitment of a right superior vena cava (SVC) to the left atrium (LA): (a) two-dimensional image; (b) Color Doppler demonstrates flow from the SVC entering both the LA (red) and right atrium (RA) (blue); (c) diagrammatic representation displaying the anatomy. The right pulmonary artery (PA) is depicted behind the SVC
Interrupted Inferior Vena Cava with Azygous Continuation
Interrupted IVC is a rare finding, occurring in only 0.6 % of patients with congenital heart defects [51]. In this entity, the portion of the IVC below the HVs is absent. The HVs drain directly into the RA, whereas the IVC connects to the azygous vein which drains to the SVC. Distal absence of the IVC is only rarely found as an isolated lesion. This anomaly is, however, common in patients with heterotaxy, and is the most pathognomonic finding in left isomerism [52].
Awareness of the caval-azygous abnormality is important: accidental ligation of the azygos vein has been reported to be fatal and venous cardiac catheterization can be troublesome in a patient with this condition [53]. The presence of IVC interruption has technical implications for venous cannulation at the time of cardiac surgery: the snare around the SVC cannula should be distal to the azygous or hemiazygous flow, if not the operative field will be flooded by the IVC flow [54]. In addition, cannulation of the hepatic veins as they enter the heart may be necessary.
It is important to diagnose this abnormality of venous anatomy in the patient with single ventricle physiology undergoing staged surgical palliation. The Kawashima operation, in which the flow from the SVC and the azygous continuation are routed to the pulmonary arteries via a bidirectional cavopulmonary anastomosis, results in diversion of 75 % of systemic venous return to the pulmonary arterial tree. The pulmonary vascular resistance must be able to accept this volume load at the time of surgery, and for this reason the cavopulmonary connection may be delayed in the patient with an interrupted IVC [15]. The exclusion of hepatic venous return from pulmonary blood flow may lead to development of diffuse pulmonary arteriovenous malformations (AVMs). Subsequent inclusion of HVs into pulmonary blood flow via an extracardiac conduit or lateral tunnel has resulted in resolution of the AVMs [55].
Transesophageal Echocardiography
Transesophageal imaging of this entity must be directed. When an attempt to view the IVC in its long axis as it enters the RA (Video 6.4) is not successful, search for the azygous vein should ensue (Fig. 6.15, Video 6.14). The azygous continuation can be visualized in the retrocardiac position thusly: one may begin by searching in the transverse or horizontal plane (0°) for a venous structure traveling in a paravertebral gutter. Alternately, a search for a vascular structure coursing in the longitudinal (90°) plane of the posterior chest (i.e., looking away from the heart, towards the spine) with cranially directed venous flow would suggest an azygous vein (Fig. 6.16, Video 6.15). Ideally the entrance of the azygous vein to the SVC should be visible and one might expect that the plane of imaging would be at 90° (ME Bicaval view). However, due to the orientation of the esophagus (parallel and to the left of the azygous course), and to the close proximity of the right mainstem bronchus, imaging of this structure may be less than rewarding. Figure 6.17 and Video 6.16 demonstrate a view of the azygous as it travels towards the SVC. At 90°, the distal azygous can be displayed coursing over the RPA seen in its short axis. This relationship between azygous and RPA also can also be appreciated from at the level of the mid esophageal ascending aortic short axis (ME Asc Ao SAX) view, with the multiplane angle at 0°, and with the probe withdrawn and turned clockwise. The RPA is first demonstrated, then the probe is slightly anteflexed to display venous flow directed away from the probe (i.e., anteriorly towards its entrance into the SVC).
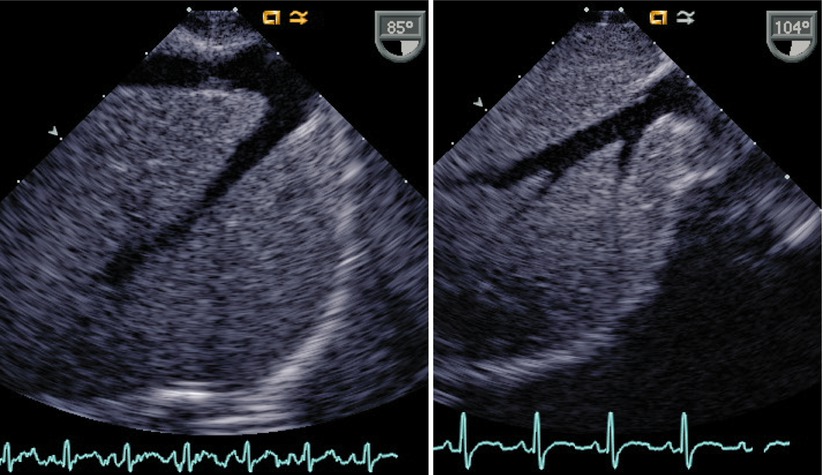
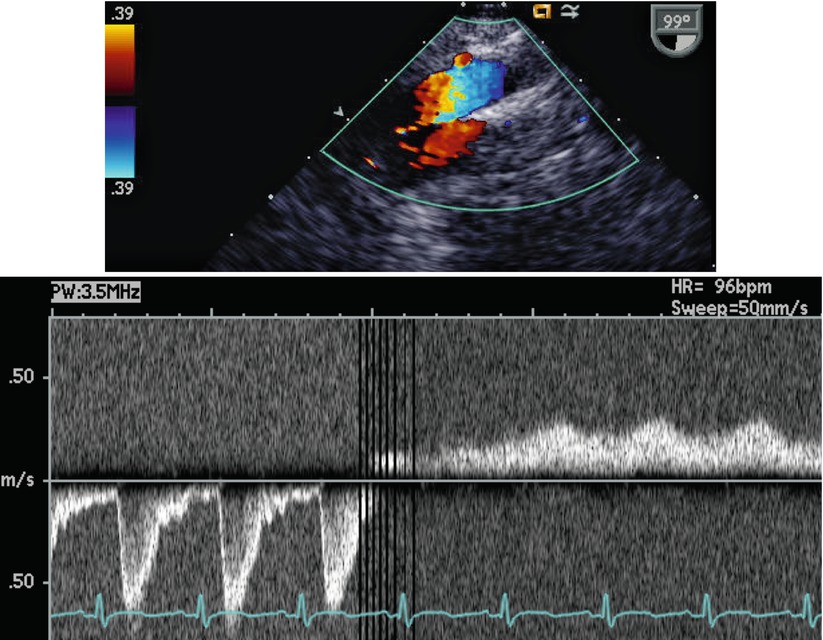
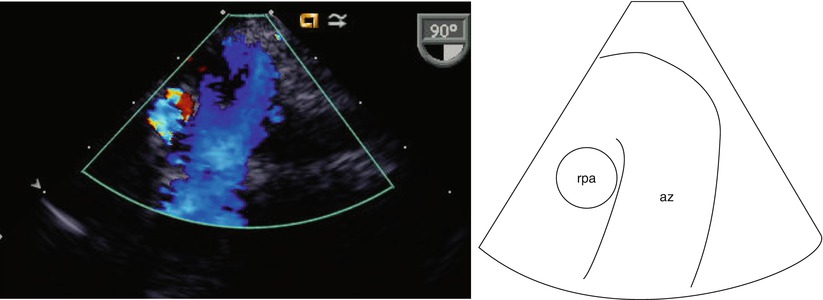

Fig. 6.15
This is a lower esophageal inferior vena cava long axis view in the sagittal plane displaying the findings in a patient with a normal inferior vena cava (left image) with hepatic veins joining this structure superiorly, in comparison to a corresponding image in a patient with an interrupted inferior vena cava (right image). In this view only hepatic veins are seen

Fig. 6.16
In the top image, the TEE probe is in a low esophageal position, but has been rotated posteriorly; the transducer is oriented in the sagittal plane. Two vascular structures are seen coursing adjacent to each other. In the lower image, the pulsed Doppler sample has been moved from the descending aorta, which has pulsatile flow directed caudally, to the azygous vein that has venous flow traveling cranially

Fig. 6.17
View of the azygous (az) vein as it travels towards the superior vena cava. At 90°, the distal azygous vein can be seen coursing over the right pulmonary artery (rpa) seen in its short axis. This is a modified view obtained at the level of the mid esophagus
Inferior Vena Cava Connection to Left Atrium
A left-sided IVC occurs with an incidence of 0.2–0.5 % [56]. Case reports include cases where the hepatic venous drainage is with the IVC to the LA [57], or is separate. In the latter situation, part or all of the abdominal portion of the right IVC is absent, but the intrathoracic segment which connects the common hepatic vein to the RA is present [58]. Blood from the lower part of the body drains to a left-sided IVC. At the level of about T12, the left sided IVC may:
Transesophageal Echocardiography
Although it may not be feasible to fully characterize this anomaly by the transesophageal imaging approach, comprehensive scanning guided by color Doppler flow interrogation may assist in this assessment.
Pulmonary Venous Abnormalities
Partial Anomalous Pulmonary Venous Connection
Partial anomalous pulmonary venous connection (PAPVC) occurs infrequently, with an incidence of less than 0.4 % in autopsy series [62]. Most commonly, PAPVC is associated with an ASD, either secundum or sinus venosus type in >75 % of cases [63–65]. PAPVC is more common in the setting of a sinus venosus defect—occurring in up to 85 % of cases, compared to a 10–15 % incidence in patients with secundum ASDs [63, 65].
The physiologic disturbance caused by PAPVC depends on the number of anomalous pulmonary venous connections involved, site of the connection, status of the pulmonary venous bed, and the presence of an ASD or other CHD. When a pulmonary vein connects anomalously to the RA or SVC, blood is preferentially shunted to this anomalous vein because of the lower RA pressure, compared with LA pressure, and can produce significant right-sided volume overload.
By far the most common type of PAPVC is right-sided, occurring in up to 89 % in series reviews [8, 64, 65]. The most common sites for drainage of anomalous right pulmonary veins are the RSVC (36–52 %) and RA (12–52 %) [8, 66, 67]. Anomalous right pulmonary veins more commonly involve two or more lobes (75 %) than single lobes (25 %) [68]. When one or both left pulmonary veins drain anomalously, they may drain into the CS, or azygous vein, yet most commonly they drain to a left vertical vein before entering the left aspect of the LIV.
One anatomic factor that affects detection of this defect is the site of the anomalous pulmonary venous drainage; anomalous pulmonary veins draining into the RSVC and entering the SVC higher than 2 cm above its entrance to the RA may be difficult to detect [68].
Transesophageal Echocardiography
Detection rates for partial anomalous pulmonary venous drainage by TEE vary from 60 to 93 % [64]. The presence of four normally draining pulmonary veins into the LA does not exclude anomalous additional or accessory pulmonary veins [8]. With anomalous pulmonary venous drainage (either partial or total), color flow Doppler evaluation is an essential part of the examination, though Nyquist limits might need to be adjusted to highlight the anomalous venous drainage.
Anomalous right pulmonary veins draining to the SVC are often best visualized by imaging the SVC-RA junction at a multiplane angle of 0° (which transects the SVC in the horizontal plane), and withdrawing the imaging probe while observing the transition from high RA to the round shape of the SVC. This is performed by initially obtaining the ME Asc Ao SAX view. A change from this round shape to a teardrop appearance, suggests the point of entry of an anomalous right pulmonary vein into the SVC (Fig. 6.18, Video 6.17) [8]. Similarly, anomalous venous connections to the free wall of the RA can be assessed in this 0° plane by rotating rightwards and advancing the probe inferiorly towards the IVC.
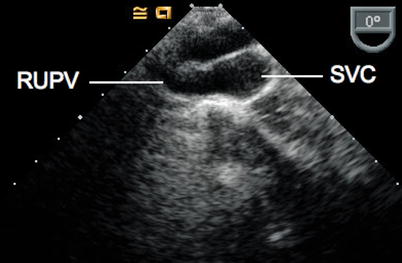

Fig. 6.18
From the mid esophageal ascending aortic short axis view with probe rotation to the right, multiplane angle 0°, the right upper pulmonary vein (RUPV) can be seen entering the superior vena cava (SVC). In real time imaging, the change in shape of the SVC from round to a teardrop shape is an indication that the patient has an anomalous connection of the RUPV to the SVC
The most common anomalous connection for PAPVC is for one or more left pulmonary veins to enter a left vertical vein draining superiorly into the LIV. The LIV empties into the SVC, which may be dilated if the shunt volume is significant. To assess the anomalous drainage, the left pulmonary veins are imaged longitudinally at about a multiplane angle of 120° as they course towards the LA from the lungs [8]. In this case, instead of entering the LA, the anomalous pulmonary veins will enter a vessel lateral to the LA, which courses anteriorly to the LPA, and is headed in a cephalad direction.
Alternately, one can screen for entry of the left vertical vein into the LIV [8]. To image the LIV, as previously described, from the ME 4 Ch view, the transducer is rotated leftwards beyond the cardiac window, towards the patient’s spine, until the Desc Ao SAX view is obtained. Maintaining the aorta in the center of the image sector, the transducer orientation is modified to display the Desc Ao LAX view at a multiplane angle of 90°-120°. With further probe withdrawal, the transverse arch can be more proximally imaged in the UE Ao Arch SAX view. The LIV will appear anterior to the upper thoracic aorta and transverse arch. At this point, at the level of the UE Ao Arch SAX, leftwards rotation of the transducer shaft should demonstrate any anomalous pulmonary veins and/or the vertical vein. Rightward rotation of the transducer will result in a long axis view of the SVC; with probe advancement, it becomes the ME Bicaval view. Anomalous right pulmonary veins entering the SVC can be seen with this latter view. Again, both imaging and color flow Doppler should be used for detection of anomalous pulmonary venous drainage.
Scimitar Syndrome
Scimitar syndrome is a rare condition consisting of either partial or total anomalous pulmonary venous drainage from the right lung to the IVC. This syndrome is often associated with a number of other abnormalities including a hypoplastic right lung and RPA, anomalous systemic arterial supply to the right lung from the abdominal aorta with or without pulmonary sequestration, abnormalities of the tracheobronchial architecture and of lung lobation, rightward displacement of the heart (meso- or dextrocardia), and pulmonary hypertension [69]. The anomalous vein resembles a Turkish sword, hence the combination of these findings is called “Scimitar Syndrome.”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


