Fig. 8.1
Association between surgical volume and risk-adjusted mortality by Aristotle difficulty: (a) low difficulty, ≤3 (P = 0.059); (b) high difficulty,>3 (P = 0.007) (Reprinted from Welke et al. [18], copyright 2009 with permission from Elsevier)
Pasquali et al. similarly confirmed a significant association between center volume and mortality in the higher risk patients (STS-EACTS or STAT categories 4–5) but not in the lower risk patients (STAT categories 1–3) [19].
Volume/Outcome Relationship by the Type of Procedure
Hirsch et al. used the administrative Kids’ Inpatient Database (KID) in 2008 to explore the institutional volume/outcome relationship for the Norwood and arterial switch operations (ASO), that represent the most complex neonatal cardiac procedures [20]. They demonstrated that in-hospital mortality significantly decreased for both the ASO and the Norwood procedure as institutional volume increased. For ASO, mortality rates were 9.4 % for institutions performing two ASOs per year, 3.2 % for 10 ASOs/year, and 0.8 % for 20 ASOs oer year; for Norwood procedure, these rates were 34.8 % for two Norwood procedures per year, 25.7 % for 10 Norwood procedures/year, and only 16.7 % when 20 Norwood procedures were done per year.
Interestingly, Karamlou et al. showed in a Congenital Heart Surgeons Society (CHSS) study in 2010 the impact of institutional volume on the risk-adjusted mortality after ASO or repair of interrupted aortic arch, but not after a Norwood procedure or repair of a pulmonary atresia with intact ventricular septum [21]. The absence of a strong volume/outcome association in regards to the Norwood procedure in this study by Karamlou et al. was not confirmed in following studies that specifically investigated this topic. Finally, the same group investigated the volume/outcome relationship in 2013 after extracorporeal membrane oxygenation (ECMO) in patients younger than 20 years, using the Project Kids’ Inpatient Database [22]. After adjustment to case complexity (RACHS-1 categories), the lower ECMO volume remained a significant determinant of in-hospital death (OR = 1.75; CI:1.03–2.94).
Volume/Outcome Relationship and the Norwood Procedure
Several recent studies have investigated the volume–mortality relationship specifically for the Norwood procedure because of the high level of system knowledge and coordination that this procedure requires. Welke et al. demonstrated that programs that do over 350 cases per year outperformed all other volume groups for the Norwood procedure [18] (Fig. 8.2).
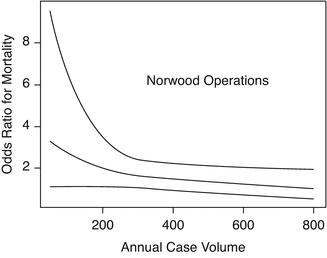

Fig. 8.2
Association between hospital volume and risk-adjusted mortality for Norwood operations (P < .001) (Reprinted from Welke et al. [18], copyright 2009 with permission from Elsevier)
Checchia et al. showed using the Pediatric Health Information System database including 801 Norwood procedures, that the survival after the Norwood procedure was associated with institutional Norwood procedure volume (p = 0.02) [23]. Hirsch et al. evaluated 624 Norwood patients in the Kids’ Inpatient Database and confirmed this significant inverse association between volume and mortality (35 % in low-volume centers versus 17 % in high-volume centers) [20].
A 2010 study by Karamlou et al. called the volume/outcome relationship into question [21]. The authors explained the absence of such a relationship in their study by three factors. First, the higher dependence of outcomes after Norwood procedure on preoperative and postoperative care, compared to the arterial switch operation; second, the higher anatomic heterogeneity of hypoplastic left ventricle compared to TGA; and third, the fact that this study missed the learning curve effect in the Norwood cohort compared to the arterial switch cohort. Moreover, the volume estimates in this CHSS study were based on the number of patients from each center enrolled in a cohort of patients with aortic atresia or stenosis selected for a Norwood operation, and not on the overall number of patients at each center undergoing the Norwood operation.
In 2012, Pasquali et al. demonstrated in a study using a large multicenter registry (The Society of Thoracic Surgeons Congenital Heart Surgery Database) that, after adjustment for patient characteristics, a lower Norwood center volume remained modestly but significantly associated with higher in-hospital mortality when evaluated as a continuous and categorical variable (OR = 1.54 (1.02 to 2.32), p = 0.04) [24]. Such a relationship did not vary significantly across preoperative risk tertiles but did not hold true across all centers (Fig. 8.3). Indeed, there are some middle volume centers with Norwood mortality rates comparable to those of higher volume centers, and some higher volume centers with mortality rates similar to those of lower volume groups. Finally, this study showed that the Norwood volume explained an estimated 14 % of the between-center variation in mortality observed after this procedure, and that the majority of between-center variation in mortality remained after adjusting for Norwood volume (p < 0.001). Based on these results, the authors concluded that the use of institutional volume alone is not a good quality metric for the Norwood procedure, and, that we would be better off to rely on center-specific risk adjusted outcomes.
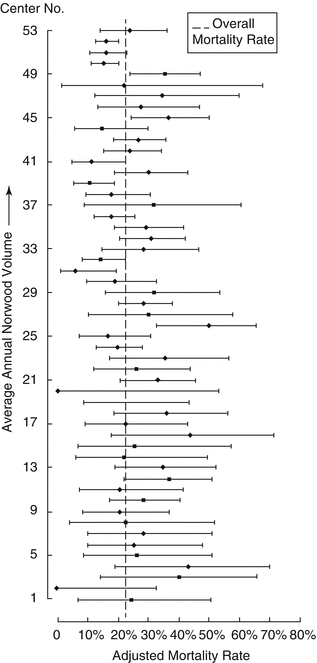

Fig. 8.3
Adjusted mortality rate displayed by increasing center volume (Reprinted from Pasquali et al. [24], copyright 2012 with permission from Elsevier)
Institutional Volume, Surgeon Volume or Volume-Independent Center Effect?
Relative Impact of Surgeon and Center Volume in Pediatric Cardiac Surgery
Studies in adult cardiac surgery have concluded that the observed insitutional volume/ mortality association was largely mediated by individual surgeon volume [25]. It has even been suggested in adult vascular surgery that a means to improve one’s chances of survival would be to select a surgeon who performs a specific operation frequently [26, 27]. In pediatric cardiac surgery, this issue was investigated by 4 groups. In 1998, Hannan et al. showed, using a New-York State clinical database, that surgeons with pediatric cardiac surgical cases volumes of less than 75 per year had significantly higher mortality rates (8.77 %) than surgeons with surgical volumes more than 75 cases per year (5.90 %) [10]. But this result was not confirmed by 2 subsequent studies that addressed this issue in the specific population of patients that required a Norwood procedure. Indeed, Checcia et al. found using a large administrative database that surgeon volume was not associated with patient outcomes after a Norwood procedure [23]. In 2010, the CHSS study by Karamlou et al. showed that neither center nor surgeon volume were associated alone with Norwood outcomes [21]. The results of these two studies might have been limited by the use of administrative data and the methodology used for calculating surgical volume.
More recently, Hornik et al. evaluated the relative impact of surgeon and center volume on mortality in a large Norwood cohort, using the Society of Thoracic Surgeons Congenital Heart Surgery Database [28]. They showed that, when analyzed individually, both lower center and surgeon volumes were associated with higher in-hospital mortality (odds ratio for surgeons with 0–5 cases versus surgeons with more than 10 cases per year = 1.60). This surgeon volume/mortality association after Norwood procedure was true in all center volume strata: lower volume surgeons had higher adjusted in-hospital mortality rates across low, medium, and high volume centers. A low-volume surgeon’s outcomes were worse regardless of center volume, but the surgeons’s results were mitigated by a large center volume. These results have been reproduced most potently in a recent analysis of the Single Ventricle Reconstruction trial, which also showed a significant survival advantage for high-volume surgeons [29]. This association can be easily understood as it has been shown that surgical technical performance improves outcomes irrespective of preoperative physiologic status or case complexity in the Stage 1 norwood procedure [30] and in other neonatal cardiac surgical procedures [31, 32]. These data could lead to the development of regional collaboration and centralization policies within and across centers through enhanced mentoring program by the highest-volume surgeons. Nevertheless, this impact of surgeon volume on Norwood mortality demonstrated by Hornik et al. was less strong when compared to the impact of surgeon volume in adult cardiac surgery [25]. This could be explained by the key role played by other providers, human factors and hospital-related factors impacting on the preoperative and postoperative management of complex single-ventricle physiology, thus decreasing the direct consequences of the impact of surgeon volume in pediatric vs adult cardiac surgery.[33]
A Volume-Independent Center Effect?
Recent studies have demonstrated that a volume-independent center-effect seems to contribute substantially to the between-center variability in outcomes. This center effect was has been demonstrated after orthotopic heart transplantation [34]: Kilic et al. demonstrated that institutional volume alone only accounted for 16.7 % of the variability in mortality between centers, and that a significant between-center variability persisted after adjusting for this factor (P < 0.001). This finding was confirmed in pediatric cardiac surgery in 2013 by Vincour et al. [35]. Vinocur et al. aimed at characterizing the relative contribution of patient factors, center surgical volume, and a volume-independent center effect on early postoperative mortality in a retrospective cohort study of North American centers in the Pediatric Cardiac Care Consortium. Although the center volume was inversely associated with outcome in all age groups and risk categories (except the lowest one), a volume-independent center effect contributed substantially more to the risk model than did the volume.
Another group revealed the impact of prior hospital performance on the current outcomes after surgery for congenital heart disease [36]. They demonstrated using the Pediatric Health Information Systems database, that prior hospital postoperative mortality was significantly associated with mortality across all risk strata of congenital heart surgery, whereas, prior hospital surgical volume tended to be associated with improved mortality after only higher-risk operations. These intriguing recent results suggest that center-specific variation in outcomes after pediatric cardiac surgery is only partially explained by operative volume and that other factors have yet to be clearly identified.
Controversies and Perspectives
The Volume Alone as a Quality Metric?
The results of the most recent previously mentioned studies demonstrate that a relationship between case volume and mortality should be interpreted with caution. The trend for lower mortality at larger centers is not universal: all larger programs do not perform better than all smaller programs. Morevover, it has been shown that the volume accounted for only a small proportion of the overall between-center variation in outcome [24, 35, 36]. The lack of long-term follow-up (beyond 30 days) in most of these studies also limits the evaluation to the very early mortality. This serious challenge prevents the authors from addressing the long-term mortality, morbidity, functional status, and neurologic status which is quite significant in single vessel pathologies even after repair [18]. Thus, the center or surgeon volume alone may not be reliable enough to measure and compare center outcomes. The use of center-specific risk adjusted outcome as a proxy tool for quality assessment may be more reliable than relying upon volume alone [17, 37]. Such an adjustment should consider at the minimum both surgical case complexity and patient specific factors [24]. Indeed, a patient’s risk factors and their level of disease severity may play a more important role in determining their individual outcome than the impact of the program’s volume.
The Confounding Bias of the Volume Factor
The true mechanism of the volume/outcome association remains controversial. Higher volume centers probably have other organizational, logistical, technical and/or human characteristics that at least partially explain this relationship. These factors include the availability of highly equipped operating rooms and cath labs, better management of health resources, ergonimic design and deployment of new technologies, composition of the care team, advanced training programs, improved preoperative and intraoperative care, multidisciplinary discussions, the use of standardized management protocols, and better resilience and timely recognition and treatment of complication [18, 19, 28, 38–41]. That suggests that higher center volume may be a surrogate for other aspects of care that are more likely to be provided at larger centers. These process measures and structural characteristics of systems that lead to better outcomes are not currently captured in available databases. These aspects including the role of human factors, team training and debriefing and non technical team skills should be extensively studied to determine their respective roles in outcomes after pediatric cardiac surgery [33, 42, 43]. The increasing mobility of skillful and experienced surgical, anesthesia and ICU staff should also be taken into account when studying the volume/outcome relationship [44]. Finally, we could also wonder whether high case volumes may lead to the improvement of outcomes thanks to an increased practice or better results attract more referrals, thus leading to higher volumes [11]. The relation between high volume and better outcomes remains strong and persistent in the field of pediatric cardiac surgery. [24, 28, 29], What then should policy makers do? what should parents and healthcare mangers do? and in view of the results of the latest studies [45, 46].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


