(1)
Department of Cardiothoracic Surgery, Glenfield Hospital, Groby Road, Leicester, LE3 9QP, UK
(2)
Division of Cardiothoracic Surgery and Center for Aortic Disease, Scott & White Clinic, Texas A & M Health Sciences Center College of Medicine, 2401 S. 31st Street, Temple, TX 76508, USA
Abstract
The most common causes of aortitis are the large-vessel vasculitides [giant cell arteritis (GCA) and Takayasu arteritis], although aortitis also is associated with systemic lupus erythematosus, rheumatoid arthritis, the HLA-B27-associated spondyloarthropathies, anti-neutrophil cytoplasmic antibody-associated vasculitides, Behçet’s disease, Cogan syndrome, and sarcoidosis. Infectious causes include tuberculosis, syphilis, salmonella, and other bacteria. Acute presentation includes aneurysm rupture, Stanford type A dissection with severe aortic regurgitation, stroke, and myocardial infarction [1, 2]. Isolated aortitis (IA) is a newly recognized condition (i.e., no associated rheumatologic or infectious disease is present), but its differentiation from Takayasu arteritis (TA) is still a challenge.
Introduction
The most common causes of aortitis are the large-vessel vasculitides [giant cell arteritis (GCA) and Takayasu arteritis], although aortitis also is associated with systemic lupus erythematosus, rheumatoid arthritis, the HLA-B27-associated spondyloarthropathies, anti-neutrophil cytoplasmic antibody-associated vasculitides, Behçet’s disease, Cogan syndrome, and sarcoidosis. Infectious causes include tuberculosis, syphilis, salmonella, and other bacteria. Acute presentation includes aneurysm rupture, Stanford type A dissection with severe aortic regurgitation, stroke, and myocardial infarction [1, 2]. Isolated aortitis (IA) is a newly recognized condition (i.e., no associated rheumatologic or infectious disease is present), but its differentiation from Takayasu arteritis (TA) is still a challenge.
Most cases of aortitis are non-infectious; however, the possibility of an infectious nature exists, and the treatment regimens widely diverge. In bacterial aortitis, a segment of the aortic wall with preexisting pathology, such as an atherosclerotic plaque or aneurysm sac, is seeded by bacteria via the vasa vasorum [3]. Similarly, tuberculous aortitis, a common problem in the developing world, may occur as a result of direct seeding of the thoracic aorta from adjacent infected tissues such as infected lymph nodes or lung lesions or by miliary spread [4].
Given this, the most common causes of noninfectious aortitis are the large-vessel vasculitides GCA and Takayasu arteritis. Although the epidemiological and clinical features of these two disorders are distinct (see Clinical Presentation), there may be significant overlap in histopathological findings (Fig. 44.1). Both GCA and Takayasu arteritis are associated with an inflammatory cellular infiltrate of the aortic media, adventitia, and vasa vasorum that contains a predominance of lymphocytes, macrophages, and multinucleated giant cells [4, 6]. Over time, scarring of the aortic media and destruction of the elastic lamina occur [6].
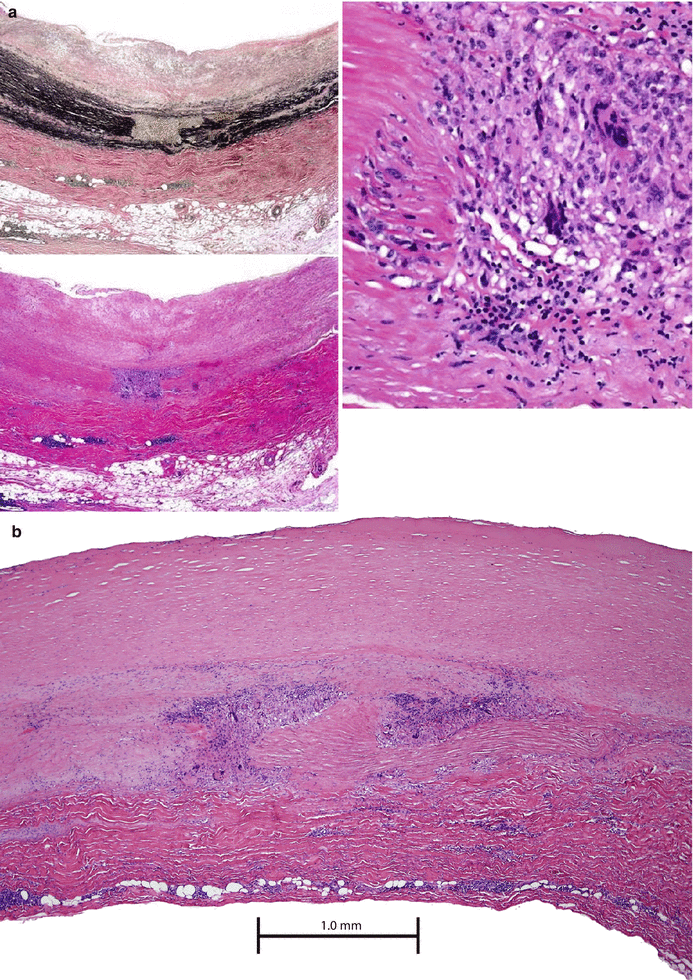

Fig. 44.1
(a) Giant cell aortitis shows more active aortitis with similar (but less advanced) medial damage and a punched out area seen in the elastic stain (top left). This area corresponds to an area of inflammation (blue cells) in the H&E slide (bottom left). The right side image shows what type of inflammation this is (lymphocytes, macrophages multinucleated giant cells). (b) Takayasu arteritis. The pathogenesis of this disease is not well understood. It’s generally thought to be autoimmune, but the antigen is unknown. The accepted theory is that the small vasa vasorum that supplies the medial smooth muscle cells (thought to be the caretakers for the elastic scaffolding) are damaged by autoantibodies or self-attacking T cells leading to ischemia in the media and death of the smooth muscle cells. The giant cell inflammation comes in to clean it up, and then collagen scar tissue replaces the elastic tissue in the media causing dilatation and wall thinning. The mechanism is described by Weyand et al. [5]
A few pathological features can be used to distinguish Takayasu arteritis and GCA. Takayasu arteritis is more commonly associated with extensive intimal and adventitial fibrosis or scarring with resultant luminal narrowing, whereas GCA is more commonly associated with extensive medial inflammation and necrosis and the formation of aortic aneurysms [5, 7]. GCA also is characterized by focal arterial inflammation, and “skip lesions” are common, a finding that can lead to false-negative findings on temporal artery biopsy in patients with associated temporal artery aortitis.
The pathogenesis of both GCA and Takayasu arteritis is unknown. Both are thought to be antigen-driven cell-mediated autoimmune processes, although the specific antigenic stimuli have not been identified [6]. Both GCA and Takayasu arteritis have been associated with specific HLA-linked antigens and a resultant genetic predisposition. In a significant percentage of cases, the diagnosis of aortitis is an incidental histopathological finding because no rheumatologic disorder, infection, or symptoms are attributable directly to the aortitis [7, 8]. Such cases of idiopathic isolated aortitis typically are localized to the ascending thoracic aorta and occur in association with ascending aortic aneurysm. Patchy necrosis of the aortic media is the primary histological finding, along with an inflammatory cellular infiltrate, which may include multinucleated giant cells (Fig. 44.2).
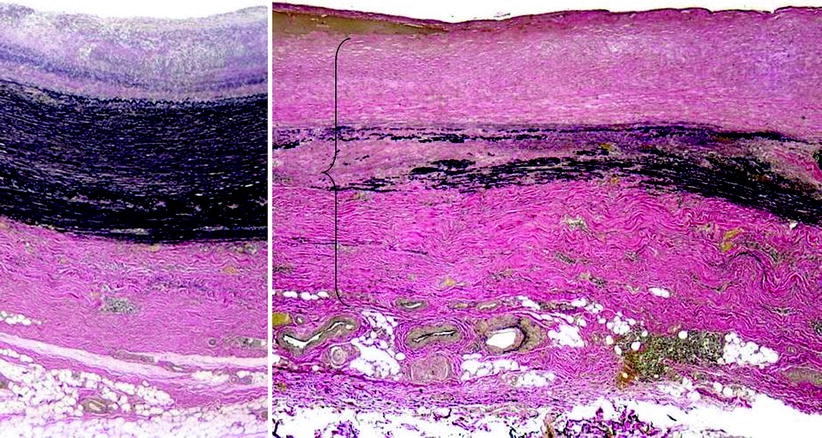

Fig. 44.2
The left hand side is taken from the better-preserved or more normal part of the specimen showing the medial layer with a relatively intact elastic scaffolding
Epidemiology
Arteritis was first described clinically in 1890 by Hutchinson [9] and histologically by Horton and associates in 1932 [10] after biopsy of a patient presenting with a swollen, painful temporal artery. The general population prevalence has been reported to be 1 % in a large autopsy series [11], whereas an average annual incidence of 17/100,000 person-years in the local population has been described [12, 13]. Although comparable data on the incidence of aortitis in Western populations or adults are not available, the epidemiology of the large-vessel vasculitides GCA and Takayasu arteritis, the most common causes of aortitis, has been studied in some depth.
Reports have suggested that in the Olmsted County, Minnesota, population, the average age- and sex-adjusted incidence of GCA among individuals ≥50 years during a 50-year period was 18.8 per 100,000 per year [14]. The overall incidence of GCA increased substantially during the 50-year follow-up period of the study, perhaps because of advances in diagnostic modalities. In this cohort, the incidence of GCA was > twofold higher among women than men (24.4 versus 10.3 per 100,000 per year, respectively), and the mean age at the time of diagnosis was 75 years [14]. Few published data are available on the epidemiology of GCA in non-Western countries, although one Japanese study reported a very low prevalence of 1.5 per 100,000 individuals ≥50 years of age [15].
The epidemiology of Takayasu arteritis is not as well characterized as that of GCA. Data from Olmstead County, Minnesota, based on a very small number of cases, estimated an incidence of 2.6 per 1 million residents per year, which is higher than that reported in any other epidemiological series [16]. Although originally described by the Japanese ophthalmologist Dr. Mikoto Takayasu, no data support an increased incidence of this disorder in Japan [17]. In a Cleveland Clinic case series of 1,204 consecutive pathological specimens taken from patients who underwent aortic surgery over 20 years, the prevalence of aortitis on surgical pathology specimens was 4.3 % [8]. Most of the surgeries were performed for aortic aneurysmal disease. In nearly 70 % of patients with evidence of aortitis, no underlying systemic disease was found, and vascular inflammation was a truly incidental finding in most of these cases of isolated idiopathic aortitis [8].
Clinical Presentation
Clinically, presentation of aortitis varies from back or abdominal pain with fever to acute severe aortic insufficiency and to an incidentally identified large thoracic aortic aneurysm. Acute aortic syndromes, including aortic dissection and rupture, can occur in persons with aortitis [18]. Inflammation-associated thrombus formation in the aortic lumen with peripheral embolization also has been reported [19]. The location of aortic inflammation (e.g., ascending thoracic versus abdominal aorta) and the presence of coexisting arteritis in other blood vessels also determine the clinical presentation. Because of the varied presentations of aortitis and the often nonspecific nature of its symptoms and signs, the index of suspicion of the evaluating clinician must be high to establish an accurate diagnosis in a timely fashion. GCA clinically presents as headache, temporal artery abnormalities on physical examination, and elevated markers of inflammation in an older adult. Common manifestations of GCA include polymyalgia rheumatica, scalp tenderness, jaw claudication (resulting from involvement of the branches of the external carotid artery), visual field changes (caused by involvement of the ophthalmic, posterior ciliary, or retinal arteries), and mononeuropathy or polyneuropathy [20]. Coronary GCA, manifest as tapering lesions in the coronary arteries and myocardial infarction, also has been reported [21]. The frequency of aortic involvement in GCA is not known. It is suggested that all patients with temporal GCA who present with symptoms suggestive of extracranial vascular involvement undergo an imaging study to evaluate the aorta and large vessels [20]. An association has been found between a history of GCA and the development of aortic aneurysm, particularly thoracic aortic aneurysm, as a manifestation of extracranial involvement [18]. In the Olmsted County cohort, it is reported that 18 % of patients with GCA and aortic aneurysm were diagnosed with thoracic aortic aneurysm at the time of diagnosis in this population, and most developed aneurysm during follow-up a median of 5.8 years after the initial diagnosis. More than half of the patients with GCA-associated thoracic aneurysm died of acute aortic dissection. In this cohort, GCA also was associated with a >twofold increased (relative risk, 2.4) risk of developing abdominal aortic aneurysm a median of 2.5 years after initial presentation with GCA [13].
Risk factors for the development of aortic and large-vessel complications in GCA have been identified, including the presence of a murmur of aortic insufficiency at diagnosis, concomitant hyperlipidemia, and coronary artery disease [18]. Presentation with classic cranial symptoms and signs of temporal arteritis (i.e., headache, scalp tenderness, abnormal temporal artery pulsations, elevated erythrocyte sedimentation rate) was a negative predictor of an aortic complication [18]. Evidence of GCA also has been identified on histopathological specimens of patients undergoing thoracic aortic aneurysm repair, including those not known to have aortic involvement [7].
In contrast to GCA, Takayasu arteritis is observed to be a much rarer disorder with a predilection for young women. The average age at diagnosis is 25–30 years, and anywhere from 75 to 97 % of patients are female [22, 23]. The most common presentation of Takayasu arteritis includes symptoms resulting from arterial occlusive disease of the aorta, aortic arch, and large vessels. Other common names for Takayasu arteritis, including pulseless disease and aortic arch syndrome, reflect its clinical presentation. Nearly all patients with Takayasu arteritis either present initially or ultimately develop large-vessel manifestations of the disease, including hypertension caused by suprarenal aortic or renal artery occlusive disease, pulse deficits and/or vascular bruits, and upper- and/or lower-extremity claudication [22]. A comprehensive vascular examination, including measurement of blood pressure in both arms and palpitation and auscultation of pulses in all major vascular regions, is a critical component of the clinical evaluation of all patients with suspected Takayasu arteritis. In addition, we recommend measuring blood pressure in all four extremities for such patients. Aortic involvement in Takayasu arteritis is very common, with angiographic abnormalities demonstrated on aortography in nearly all patients [22, 23]. The abdominal aorta is the most common site of involvement, followed by the descending thoracic aorta and aortic arch [22, 23]. At the time of aortography, stenotic lesions in the aorta are most frequently detected, although aortic aneurysm also is common and has been reported in up to 45 % of patients in published case series [22, 23]. Case series also have reported rapid aortic aneurysm expansion, aortic rupture, and the development of aortic aneurysm at the site of anastomoses of prior reconstructive surgery among patients with Takayasu arteritis [24, 25]. Nearly 40 % of patients with Takayasu arteritis develop cardiac abnormalities, including acute myocardial infarction, angina pectoris, and acute aortic insufficiency. In these cases, the cardiac pathology is related directly to the aortic inflammation, including aortic insufficiency as a result of aortic root dilatation and coronary ostial stenoses resulting from aortitis (Fig. 44.3).
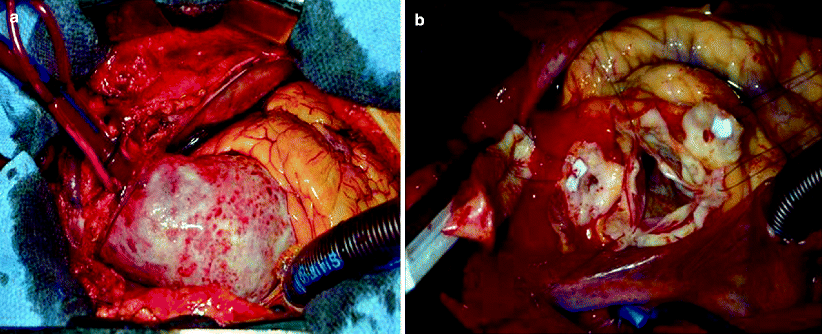

Fig. 44.3
A 7-cm ascending aortic aneurysm involved with giant cell aortitis. Note the glistening cobblestone appearance. Inside view of the coronary buttons and residual involved aorta in a patient with aortic root involvement who was prepared for a valve-preserving aortic root reconstruction. The inflammatory process is grossly evident in the residual aorta, but the morphology of the valve cusps is preserved
Diagnostic Testing
When the diagnosis of aortitis is suspected on the basis of clinical presentation, expedient imaging of the entire aorta with an appropriate modality is critical to establish the diagnosis. Modern imaging tools for the aorta include CTA, MRA, and ultrasonography. CTA and MRA have the advantage of imaging the components of the aortic wall and periaortic structures rather than the lumen only, as is the case of conventional angiography. Invasive aortography generally is reserved for cases in which diagnosis of an acute aortic syndrome is uncertain despite noninvasive imaging or for performance of catheter-based revascularization procedures in select patients. Positron emission tomography (PET) scanning has emerged for targeted imaging of vascular inflammation and may be particularly useful when combined with traditional cross-sectional imaging modalities. CT in the setting of acute aortitis may demonstrate thickening of the aortic wall and periaortic inflammation, although milder degrees of inflammation or wall edema may not be apparent. MRA, generally with gadolinium contrast enhancement, is emerging as a noninvasive imaging modality of choice for aortitis, particularly aortitis associated with GCA and Takayasu arteritis. MR imaging (MRI) may be used to image the entire aorta without radiation exposure or iodinated contrast, and it provides excellent resolution of the aortic wall. Recently, the use of 18-fluorodeoxyglucose (18F-FDG) PET, either alone or in combination with contrast-enhanced CTA or MRA, has emerged as a potential tool for the initial diagnosis and assessment of disease activity of aortitis caused by either GCA or Takayasu arteritis. Recent imaging series have reported a sensitivity of 60–92 % and a specificity of 88–100 % of 18F-FDG PET for diagnosing active inflammation in arteritis, but these studies have been limited by small sample size, heterogeneous patient population, and inconsistent choice of a reference standard [26, 27].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


