Age, race, gender
Behavior
Disorders
Treatments
Old > young
Prolonged travel
Cancer
Surgery
Blacks > whites
Sedentary lifestyle
Antiphospholipid antibody syndrome
Chemotherapy
Men > women
–
Polycythemia vera
Hormone therapy
–
–
Genetic mutations
–
Studies of autopsy data estimate that 10–30% of VTE cases are fatal within 30 days. PE is the primary cause of mortality in those with VTE. Approximately 20–25% of all PE cases present as sudden death [14–17]. It is considered a common cause of death in hospitalized patients (15% of total in-hospital mortality) that may be prevented with timely diagnosis and appropriate use of prophylaxis [5, 8, 13]. Considering that PE is fatal in only about 6.5% posttreatment [17], missing its diagnosis in the setting of rapid deterioration may contribute to poor prognosis.
Timely restoration of hemodynamic stability has been shown to prevent mortality. In the International Cooperative Pulmonary Embolism Registry (ICOPER) , the 90-day mortality rate for patients with acute PE and cardiogenic shock at presentation (108 patients) was 52.4% versus 14.7% for the hemodynamically stable [18]. Consistently, the Germany-based Management Strategy and Prognosis of Pulmonary Embolism Registry (MAPPET, 1001 patients) showed the in-hospital mortality was 25% for those presenting with cardiogenic shock, 65% for those requiring cardiopulmonary resuscitation , and 8.1% for hemodynamically stable patients [19].
Pathophysiology of VTE
PE and DVT lie in the spectrum of VTE. Thrombi form in the deep veins of extremities, such as the calf, and then propagate into the proximal veins, where they likely embolize [7] (Fig. 31.1). Lower-extremity DVT is found in 79% of PE patients, and if not found, it likely has embolized [14]. Because of the circulation arising from both pulmonary and bronchial arteries, pulmonary infarct is uncommon.
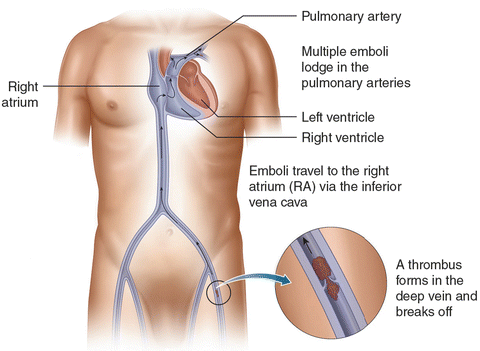

Fig. 31.1
A thrombus forms in the deep vein and embolizes to the pulmonary arteries
Acute right-sided heart failure due to increased pulmonary vascular resistance (PVR) is the main cause of death in PE [20]. The rapid increase in afterload causes right ventricle (RV) dilatation, which in the setting of systemic hypotension diminishes the coronary perfusion, resulting in cardiac ischemia. The septal shift resulting from right ventricle dilatation further reduces left ventricular (LV) preload and output, and the patient enters a worsening cycle of acute right-sided heart failure and cardiogenic shock [21] (Fig. 31.2).
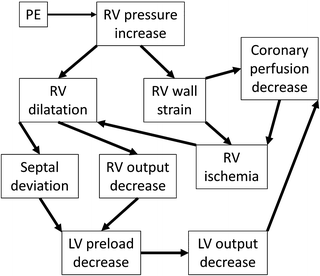

Fig. 31.2
The pathophysiology of right heart failure and cardiogenic shock due to PE
Although it has been thought that PVR increase was due to the mechanical obstruction of the pulmonary vasculature, studies have not found a correlation between the mechanical obstruction and the hemodynamic derangement associated with PE. This finding has been confirmed by only a small, insignificant rise in the pulmonary artery pressure (PAP) after cross-clamping the left or right pulmonary artery during a surgical procedure or by unilateral balloon occlusion. The increase in PAP is never enough to cause right-sided heart failure [20].
Interestingly, PE often obstructs only about 25% of the pulmonary vasculature but causes significant pulmonary hypertension and heart failure [22]. Vasoconstriction induced by PE is likely the main cause. In symptomatic, non-heparinized PE patients , stellate ganglion blockade that prevents pulmonary vasoconstriction [23] has been shown to reduce cyanosis, dyspnea, and cardiac shock [24].
PE has been characterized as massive, submassive , and non-massive based on the hemodynamic burden of the emboli. This classification is useful for guiding medical and interventional treatment decisions. Massive PE has been described as acute PE with loss of pulse, persistent bradycardia, or sustained hypotension (systolic blood pressure less than 90 mmHg for at least 15 min or requiring inotropic support). The hypotension cannot be caused by other factors, such as arrhythmia, hypovolemia, sepsis, or left ventricular dysfunction. Submassive PE is acute PE without systemic hypotension (systolic blood pressure greater than or equal to 90 mmHg) but with either RV dysfunction with RV dilation or myocardial ischemia (MI) . Non-massive or low-risk PE is acute PE that lacks symptoms of massive or submassive PE [25]. Surgical or catheter-based intervention should be considered for patients with massive or submassive PE whose hemodynamic decompensation appears imminent (Table 31.2).
Table 31.2
Massive, submassive, and low-risk PE
Massive PE | Submassive PE | Low-risk PE |
|---|---|---|
Prolonged hypotension | No systemic hypotension | No sign of massive or submassive PE |
No pulse or bradycardia | RV dysfunction or MI |
Diagnosis of PE
Patients with PE present with a variety of symptoms. They often have dyspnea or chest pain, which may be sudden or evolving over days to weeks. Many patients with PE may also have DVT symptoms, such as leg pain, warmth, or swelling in the extremities. Pleuritic chest pain and hemoptysis occur more often in those with pulmonary infarction, which is characterized by smaller, more peripheral emboli and a pleural rub. Cough, palpitations, light-headedness, fever, wheezing, and rales may result from PE or concomitant illnesses. Tachypnea and tachycardia are common but nonspecific findings.
Pulmonary hypertension due to PE may present as elevated neck veins, a loud second heart sound (S2), a right-sided extra heart sound (S3, S4), systolic murmur in the left sternal edge, a right ventricular lift, and, less frequently, hepatomegaly. These symptoms are suggestive but neither sensitive nor specific [26]. Patients with these symptoms often do not have the disease. However, PE is often unlikely in those without either chest pain or acute or worsening shortness of breath [27].
The arterial blood gas analysis often suggests hypoxia and hypocapnia. Chest radiography results are nonspecific; it is common for PE patients to have normal chest X-ray. However, an elevated hemidiaphragm, unilateral pleural effusion, and atelectasis may be seen occasionally. Chest radiography is usually useful for ruling out alternative diagnosis with more obvious findings.
With PE, the electrocardiography (ECG) may show tachycardia, nonspecific T wave and ST changes in the precordial leads, the S1Q3/S1Q3T3 pattern, or a right bundle branch block. These findings also are uncommon and nonspecific [27]. RV dysfunction may be reflected in elevated brain natriuretic peptide (BNP) or N-terminal pro-BNP. Myocardial infarction due to RV dysfunction may elevate troponin.
Due to the difficulty diagnosing PE based on clinical findings, the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) highlighted the need for determining the pretest probability to influence the posttest probability [27]. This was confirmed by Wells et al. as the Wells scoring system and application of D-dimer [28, 29]. They demonstrated a scoring system based on DVT symptoms, no alternative diagnosis, tachycardia, and risk factors, such as recent immobilization or surgery, malignancy, hemoptysis, and previous VTE [29].
Currently, in addition to the Wells criteria, there are the pulmonary embolism rule-out criteria (PERC) , the Geneva score , and the Charlotte rule for estimating the probability of PE. No difference in the rate of missed PE was observed comparing these systems [27] (Table 31.3). PE may be effectively excluded based on these assessment systems and D-dimer levels, a plasmin-derived fibrin degradation product that is highly sensitive but not specific for VTE [28].
Table 31.3
Diagnostic criteria for PE
Wells | PERC | Geneva | Charlotte |
|---|---|---|---|
DVT | Unilateral leg swelling | Unilateral leg pain | Age >50 or HR/SBP >1 |
Previous DVT/PE | Previous DVT/PE | Previous DVT/PE | Unilateral leg swelling |
Tachycardia | Tachycardia | Heart rate >74 | Surgery in <1 month |
Active malignancy or palliative | Trauma/surgery <1 month | Surgery/leg fracture <1 month | Unexplained hypoxemia |
Surgery <1 month | Age >49 | Age >65 | Hemoptysis |
Hemoptysis | Hemoptysis | Hemoptysis | D-dimer assay, if PE unlikely |
>2-day immobilization | Exogenous estrogen | Active malignancy | Imaging, if PE likely |
PE most likely | O2 sat in RA <95% | – | – |
Once a patient is suspected of PE based on these exclusion criteria, computed tomography (CT) pulmonary angiography is commonly used to diagnose PE (Fig. 31.3). For the patients with renal failure or contrast dye allergy, who cannot undergo a CT angiography, ventilation/perfusion (V/Q) scanning may be considered, at the cost of lower sensitivity [29]. To evaluate the RV dysfunction or stress due to PE, transthoracic echocardiography may be employed.


Fig. 31.3
Computed tomography pulmonary angiography of a patient with emboli in the left and right pulmonary arteries (red arrows)
Management of PE
Patients with PE and no contraindications for anticoagulation should be given subcutaneous low-molecular-weight heparin (LMWH), intravenous or subcutaneous unfractionated heparin, or subcutaneous fondaparinux. For those with heparin-induced thrombocytopenia , a non-heparin-based anticoagulant, such as lepirudin, argatroban, or bivalirudin, may be employed. For those with high probability of PE, they should be given anticoagulant during the diagnostic work-up.
Thrombolytic medications, streptokinase, urokinase, and alteplase, promote the hydrolysis of fibrin molecules , breaking down the thrombus. They are enzymes that convert the native circulating plasminogen into plasmin, a serine protease that cleaves fibrinogen and releases fibrin or D-dimer fragments. These medications have been FDA approved for treatment of massive/submassive PE and compared to heparin, offer faster alleviation of symptoms and stabilization of cardiovascular function .
At 24 h after starting treatment, heparin resulted in no significant improvement in pulmonary blood flow, but addition of fibrinolysis improved the total perfusion by 30–35%. By day 7, the blood flow increased significantly with 65–70% improvement [25]. Thus, thrombolysis is effective in cases with a larger clot burden. However, they are contraindicated in patients with bleeding risks, such as previous hemorrhagic stroke, recent major surgery, or trauma, and may result in minor to fatal hemorrhage. Therefore, they are not recommended for patients with low-risk PE or submassive PE with minor symptoms (Fig. 31.4).
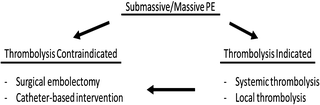

Fig. 31.4
For submassive/massive PE, surgical or catheter-based embolectomy may be considered for patients, who are contraindicated or refractory to thrombolysis
With hemodynamic instability or RV dysfunction, removal of the embolus surgically or using a catheter-based intervention is an effective alternative to fibrinolysis in patients with high bleeding risks or inadequate time to infuse thrombolytic agents. Surgical embolectomy is suited for acute PE patients with a right atrial thrombus or paradoxical embolism as well as those that are refractory to thrombolysis [30].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


