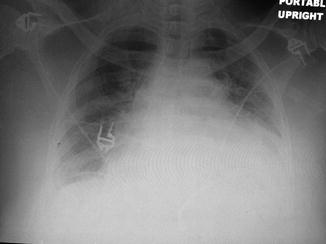
Fig. 12.1
Computerized tomography scan of the chest demonstrating a patient with delayed pericardial effusion after cardiac surgery resulting in hypotension, low cardiac output, and bilateral pleural effusions with atelectasis
Despite over 70 years experience of cardiac surgery, the question of pericardial closure after cardiac procedures has not been definitively answered [23]. Some surgeons close the pericardium, others do not, and many believe that neither is of clinical significance. There has been little scientific study and no clinical randomized trials. What is known is that the pericardium maintains compliance and the integrity of the Starling curve as it limits hypertrophy with exercise. It is structurally protective with mechanical membranous and ligamentous function. Benefits of closing the pericardium include making potential reoperation safer with fewer adhesions, return of the mediastinum back to the original setting, and the possibility of improved hemodynamics [23]. Others are concerned that closure results in increased risk of tamponade, negative hemodynamics, increased use of inotropes, and possible graft compromise [23]. Tension- free substitutes do exist but come with financial cost and possible infectious complications [23].
Aortic Dissection
Pericardial effusion and tamponade may occur in the setting of Type A aortic dissection. In this setting, surgery for the dissection should be performed immediately rather than drainage of the hemopericardium, which may result in further bleeding [4].
Pericardial Window
The etiology of pericardial fluid causing compression varies and includes infections, post-irradiation sequelae, collagen vascular diseases, myocardial infarction, and malignancy [24–30] (Table 12.1). Medical therapy may be started initially but large effusions associated with pericarditis may be unresponsive to non-steroidal anti-inflammatory drugs, corticosteroids, or colchicine [29]. The diagnosis of a large pericardial effusion can be made from CXR, computerized tomography (CT) scan or echocardiography (Fig. 12.2). The definitive treatment for large pericardial effusions or cardiac tamponade is pericardial drainage. If hemodynamic compromise has occurred, medical treatment has failed, or a diagnosis is needed, then intervention is required. The specific presence of purulent pericardial fluid may have a distinct effect separate from tamponade physiology and has a characteristically high mortality without drainage [31].
Congenital |
Congenital anomalies and defects |
Pericardial cysts |
Acute and chronic pericarditis |
Effusive +/− tamponade |
Idiopathic |
Uremic |
Infectious |
Pyogenic |
Tuberculosis |
Viral |
Neoplastic |
Associated with systemic disease (connective tissue disease) |
Traumatic |
Radiation |
Constrictive +/− effusion |
Idiopathic |
Infectious |
Previous cardiac surgery |

Fig. 12.2
Chest x-ray of a patient with a large pericardial effusion from idiopathic pericarditis. Note the marked cardiomegaly and obscured left diaphragm
An open surgical procedure offers several advantages. Firstly, complete drainage can be achieved. There is ample access to pericardial tissue for histopathological and microbiological diagnoses. Loculated or mixed effusions can be evacuated (Fig. 12.3). There is little risk of traumatic injury as there is direct visualization of the pericardial space.
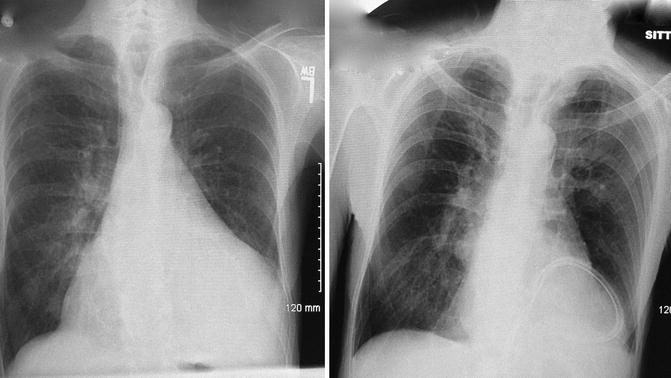

Fig. 12.3
Example of pre- and post-operative chest x-rays in a patient who underwent open surgical drainage via sub-xiphoid pericardial window. Note the position of the long flexible drain lying behind the heart and marked decrease in the large cardiac silhouette
Sub-Xiphoid Pericardial Window
Sub-xiphoid pericardial window, or subxiphoid pericardiostomy, is a common approach to pericardial drainage [25–29, 32]. General anesthesia is preferred for this procedure, but it may be performed with monitored or local anesthesia [33]. Rapid induction can proceed with careful attention to blood pressure throughout the process. The patients are placed supine during the procedure. If the patient is suspected to be in true cardiac tamponade, the patient’s chest is often prepared and draped for surgery while he remains awake. In case of hemodynamic collapse upon induction, rapid evacuation of fluid may be performed. Sub-xiphoid drainage under local anesthesia is also an acceptable choice for patients who are unstable.
Technique
A midline incision is made from the xiphisternal junction to below the tip of the xiphoid. Alternatively a 5 cm transverse incision can be made at the tip of the xiphoid [1]. The upper linea alba is divided in the midline and the xiphoid is incised or completely resected. The tissue plane between the posterior wall of the sternum and the anterior pericardium is developed by blunt finger dissection. The distal sternum is then elevated for visualization of the pericardium. The anterior pericardium is grasped directly and incised to drain the fluid. Culture swabs, fluid analysis, and cytology specimens are collected. Pericardial fluid is analyzed for hematocrit and cell count, amylase, lactate dehydrogenase, protein, glucose, culture, and cytology. The pericardial space is then evaluated by direct vision, digital exam, and echocardiographic visualization if needed. The pericardium may be explored digitally to identify adhesions or tumor deposits. The optional use of intraoperative transesophageal echocardiography may facilitate removal of complex loculated collections and can insure complete drainage. A piece of pericardium is excised, typically 4–5 cm in size (Fig. 12.4). A single chest tube is placed through the pericardiotomy, exiting the body through a separate incision. Both firm large bore and soft flexible drains may be used to insure optimal drainage, particularly in the case of a bloody effusion. The chest tube is left in place for several days after the operation until the drainage is minimal, usually <50 cc/day. This time period is the key to this procedure. The irritative nature of the chest tubes within the space can help form adhesions between the pericardium and epicardium to help prevent recurrence. The chest incision is closed in two layers and covered with sterile dressings.
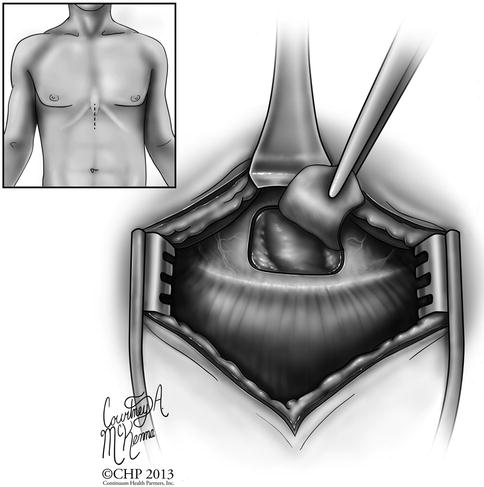
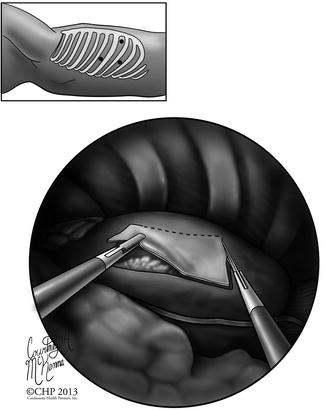

Fig. 12.4
Diagram of the incision site and technique for performing a sub-xiphoid pericardial window. Direct visual access of the pericardium and pericardial space is available

Fig. 12.5
Diagram of the incision sites and technique for performing a video-assisted thoracoscopic pericardial window. Note the visualization of the pericardium within the chest cavity and the area of pericardium available for resection
Outcomes
Multiple retrospective studies over the past 25 years have reviewed outcomes of subxiphoid pericardial window, after its initial success in providing drainage and preventing recurrent effusion. Early studies, however, reported 30-day mortality rates of up to 20 %, with deaths due to associated cancer rather than the procedure itself [34]. Current studies show 30-day mortality of 0.8–4.8 % [27, 35, 36] and a recurrence rate of 2–10 % [35–37].
Risk factors for short-term mortality include the occurrence of postoperative low cardiac output syndrome (PLCOS). This syndrome is early and rapid cardiac failure after relief of tamponade and can occur in up to 4.8 % of patients [36]. It accounted for all the postoperative deaths (0.8 %) in a recent study [27]. The mechanism for this complication is not completely clear but is likely due to chronic external support of the heart by pericardial fluid. When this support is removed, the heart may immediately overdilate, resulting in systolic dysfunction and failure. These patients need close monitoring, as the mortality from this syndrome is very high.
Complications of the procedure include recurrence and transient arrhythmias [36]. Constrictive pericarditis develops in up to 3 % of patients who survive after 1 year, most commonly in those patients with tuberculous pericarditis or non-tuberculous bacterial pericarditis [27]. Direct injury to the heart may occur in 0.8 % and requires median sternotomy for treatment [27]. Wound infections may occur in up to 5 % of patients [27].
Underlying disease, specifically malignancy, is an important risk factor for decreased survival after subxiphoid pericardiostomy [28]. The presence of malignant pericardial effusion leads to limited life expectancy; better survival is found in those patients who have malignant cells but no tumor in the pericardium [38]. The type of malignancy also plays a role: patients with hematologic malignancy were found to have significantly longer survival when compared with patients with other malignancies [36]. Metastatic lung cancer to the pericardium has been shown to have a very poor survival rate, particularly when compared to other cancers [28, 39]. Detectable malignant invasion of the thorax on CT scan and positive echocardiographic findings compatible with tamponade are two independent risk factors for poor outcome [28].
Comparison with Percutaneous Drainage
The optimal management for pericardial effusions with acute pericardial tamponade remains controversial. The two most commonly performed techniques include subxiphoid window and percutaneous catheter drainage (Table 12.2). Percutaneous catheter drainage may be performed with local anesthesia and requires a needle to be placed in the pericardial space, usually under echocardiographic guidance. A guide wire can be inserted and a drainage catheter is passed over the wire [40].
Advantages | Disadvantages | |
|---|---|---|
Subxiphoid pericardial window | • Direct visualization | • General anesthesia (or local anesthesia with sedation for unstable patients) |
• Access to pericardial tissue for histopathological and microbiological diagnoses | • More invasive | |
• Evacuation of loculated or mixed effusions | ||
• Less risk of traumatic injury | ||
Percutaneous catheter drainage | • Local anesthesia | • Higher complication rate (blind needle placement) |
• Less invasive | • Higher recurrence rate | |
• Immediate relief of symptoms |
Multiple studies have directly compared these two techniques (Table 12.3). Allen et al. performed a direct comparison of the two procedures in 1999 [35]. The mortality, complication, and recurrence rates were significantly higher for percutaneous drainage (4.3, 17.3, and 33.3 %, respectively) than for subxiphoid drainage (0, 1.1, and 1.1 % respectively) [35]. This same study combined published collected data from 1977 to 1999 and found 560 patients undergoing subxiphoid pericardial window for pericardial tamponade. These patients had a mortality rate of 0.6 %, a complication rate of 1.5 %, and a recurrence rate of 3.2 % [35]. Percutaneous catheter drainage (331 patients) demonstrated increased mortality, morbidity and effusion recurrence of 4.3, 10.6, and 13.9 %, respectively [35]. Subxiphoid pericardiostomy is a safe and durable technique for chronic effusion despite the less invasive nature of the percutaneous drain.
Date | Patient number | Mortality (%) | Morbidity (%) | Recurrence (%) | |
|---|---|---|---|---|---|
Subxiphoid | |||||
Combined (Allen) [35] | 1977–1995 | 560 | 0.6 | 1.5 | 3.2 |
Allen [35] | 1999 | 94 | 0 | 1.1 | 1.1 |
McDonald [37] | 2003 | 150 | 10.7 | 0.1 | 4.7 |
Saltzman [41] | 2012 | 72 | 19.8 | 26.4 | 2.8 |
Percutaneous | |||||
Combined (Allen) [35] | 1984–1999 | 331 | 4.3 | 10.6 | 13.9 |
Allen (included in combined) [35] | 1999 | 23 | 4.3 | 17.3 | 33.3 |
McDonald [37] | 2003 | 96 | 22.9 | 3.1 | 15.6 |
Saltzman [41] | 2012 | 121 | 18.1 | 4.9 | 28.9 |
Others studies concur that recurrent effusion is more frequent in the percutaneous versus the open group, (28.9 % vs. 2.8 %), [41] and (15.6 and 4.7 %) [37]. Mortality rates that are higher in the percutaneous group are difficult to extract from data as variable comorbid conditions and hemodynamic states leading to percutaneous drainage are confounding factors. It has been shown that extended catheter drainage may help decrease this rate of recurrence in percutaneous techniques, likely secondary to the inflammatory/adhesion forming qualities of the drains themselves [42].
Ultimately, an individualized, patient-centered approach is necessary in making decisions about percutaneous versus open procedures. Patients with positive cytology on previous pericardiocentesis or a limited lifespan where recurrence is unlikely may benefit from percutaneous drainage. Effusions that are loculated, posterior, or of mixed density, may be better served with an open subxiphoid window. The presence of malignancy, direct invasion of the pericardium, poor long term prognosis, and clinical conditions all play a role in this decision process.
Thoracoscopic Pericardial Window
An alternative technique to subxiphoid window is video-assisted thoracoscopic surgery (VATS) drainage of pericardial fluid into the pleural space. Anesthesia preparation is more complicated and time-intensive. It requires bronchoscopic-directed placement of a double-lumen endotracheal tube to achieve single lung ventilation. Lateral decubitus positioning is also required. Thoracoscopy is performed through a 10-mm camera port placed in the seventh intercostal space in the mid-axillary line. The pericardial resection and any additionally procedures are completed through one or two working incisions. A section of pericardium approximately 4–5 cm in diameter is resected anterior to the phrenic nerve, which creates a window into the pleural space [43] (Fig. 12.5). A flexible chest tube may be placed into the pericardial space along with a pleural tube. Alternatively, a single chest tube may be placed into the pleural space for drainage of both cavities.
This procedure has specific advantages and disadvantages. One benefit of the thoracoscopic approach is that it allows simultaneous access to the pleural and pericardial spaces, which is helpful in the setting of large pleural effusion or concomitant pleural disease. Thoracoscopy affords better visualization of the pleural cavity and pericardium, which allows for more direct sampling of suspicious sites [43, 44]. The entry into another body cavity is a potential disadvantage depending on the co-morbidities of the patient. Generally, sub-xiphoid windows are preferred in a setting in which the patient is unstable to avoid the prolonged anesthetic and preparation time that is associated with VATS.
In a study of 71 patients directly comparing sub-xiphoid and VATS, O’Brien et al. found significant differences in complication rates [44]. The sub-xiphoid group had a 2 % morbidity rate while the VATS group had a 27 % morbidity rate [44]. Complications included pneumothoraces, an on-going air leak requiring discharge with a Heimlich valve, and readmission for self-limited drainage from the chest tube site. The 30-day mortality rate was 13 and 0 % for subxiphoid and VATS, respectively, but all mortalities were nonspecific to the procedure and were attributed to advancing malignancy or worsening of underlying medical illness in the absence of recurrent effusion [44]. Patients with greater comorbidities were selected for sub-xiphoid drainage. Recurrence was similar in both groups (10 % of the sub-xiphoid group and 8 % of the VATS). Malignant effusions were found to have a greater risk of recurrence [44].
A recent study found that limited survival is not a contraindication for VATS pericardial window as selected patients could achieve improvement through palliation [45]. Prognostic factors of poor survival included pericardial cytology with metastatic involvement of the pericardium, similar to other studies [44, 28]. Others have found that there are few differences in recurrences or complications, but that operative time is longer for VATS [46].
In summary, the sub-xiphoid approach is simpler, faster, and slightly less morbid. This is the preferred approach if the patient’s life expectancy is likely to be limited due to major comorbidities or extensive metastatic disease. In contrast, those patients with benign disease, malignancy that has not metastasized extensively or is responsive to chemotherapy, and those who require concomitant intrapleural procedures, would benefit from VATS [44] (Table 12.4).
Advantages | Disadvantages |
|---|---|
• Simultaneous access to pericardial and pleural spaces | • Prolonged anesthesia time |
• Better visualization of pericardium and pleural cavity | – Double lumen tube placement for single lung ventilation |
• More direct sampling of suspicious sites | – Lateral decubitus positioning |
• Concomitant thoracoscopic procedures | • Increased morbidity in presence of comorbid conditions or extensive metastatic disease |
Pericardiectomy
Indications
Constrictive pericarditis is a rare but severely disabling condition of the pericardium leading to impaired filling of the ventricles and reduced ventricular function [4]. The majority of cases of constrictive pericarditis are idiopathic [4, 47]. Patients present with symptoms such as dyspnea, orthopnea, jugular venous distention, or ascites. CXR or CT scan may confirm a thickened or calcified pericardium, a classic diagnostic feature of constrictive pericarditis [4]. However constriction may present in up to 18 % of patients with normal pericardial thickness [48]. True constrictive physiology is best defined at cardiac catheterization with findings as described in previous chapters. The hallmarks of constrictive physiology are equalization of diastolic pressures in the ventricles and a dip plateau pattern (square root sign) of the ventricular filling pressure curves [1]. Pericardiectomy for constrictive pericarditis corrects hemodynamic abnormalities and can produce dramatic clinical improvement [47].
Pericardiectomy is indicated once the diagnosis of constrictive pericarditis has been established. Constrictive pericarditis is irreversible and surgical resection is the only effective treatment. Surgeons are sometimes consulted to evaluate patients for pericardiectomy who have frequent and highly symptomatic recurrences that are refractory to medical therapy [4]. Recurring pericarditis management relies on exercise restriction and is treated medically with NSAIDS, colchicine, and/or corticosteroids [4]. Patients who are deemed candidates for surgery should be on a steroid-free regimen for several weeks prior to surgery. Patients with little physiologic effects and significant comorbidities should be delayed until more significant symptoms occur. This is especially true in the case of radiation-induced pericarditis, in which the myocardial tissue is affected [1, 49].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


