Surgical Considerations in Nontransplant Surgery for Congestive Heart Failure
Indu Deglurkar
Kathy J Hoercher
Patrick M. McCarthy
The ever-increasing incidence of heart failure, coupled with poorly met demands for donor hearts, has led to the evolution of alternative surgical and device management strategies in end-stage heart failure. Medically treated patients with ischemic cardiomyopathy have a five-year survival of 28%. The most common surgical procedures currently employed in heart failure patients are discussed in this chapter. The selection of the appropriate operation in a patient is a complex decision-making process, rigorously based on pathophysiologic considerations. In this population, all factors affecting surgical risk should be carefully evaluated preoperatively and surgery should be recommended when definite benefits in survival and quality of life can be reasonably predicted. The current treatment options in patients with compensated heart failure include coronary revascularization, mitral valve repair, left ventricular reconstruction, mechanical assist device, and cell therapy (under evaluation). Patients with heart failure may require a combination of procedures, including revascularization, mitral repair, LV reconstruction, and eventually regenerative cell therapy to address all the pathophysiologic components creating the clinical picture. These procedures, combined with optimal medical therapy, help improve survival and avoid or postpone cardiac transplantation.
CABG IN PATIENTS WITH SEVERE LV DYSFUNCTION
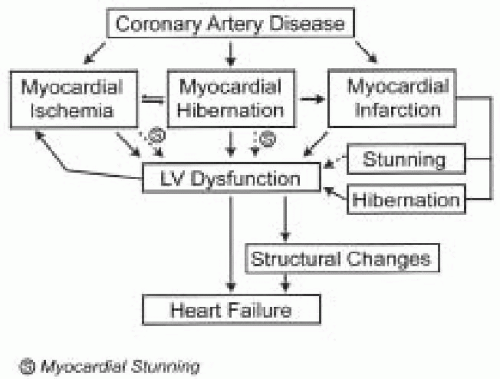
Ischemic cardiomyopathy is the most common cause of heart failure (1,2). Congestive heart failure affects 5.5 million people in the United States with an annual incidence of 550,000. Myocardial dysfunction occurs due to loss of myocytes. At a cellular level there is loss of myofibrils and disorganization of structural proteins within the myocyte. Fibrosis occurs with loss of the geometrical shape of the heart (3,4,5). The sequelae of coronary artery disease are outlined in Fig. 34.1.
The clinical presentation in these patients can be stable or unstable angina, arrhythmia, or heart failure. Evaluation includes assessment of symptoms, presence or absence of angina, NYHA class, episodes of heart failure and comorbid factors (pulmonary, renal, peripheral, and cerebrovascular disease). Coronary angiography determines the extent of disease, adequacy of distal targets, the patency of prior grafts and the presence of left ventricular aneurysm by LV angiogram that may require concomitant resection, any valvular regurgitation, and the degree of left ventricular dilatation. Myocardial viability tests with nuclear medicine tracer techniques (such as Thallium,
PET, and SPECT scan), echocardiography (resting and Dobutamine Stress echo), and MRI can be performed to assess the viability and extent of reversibility of the ischemic myocardium. Some studies have suggested that for significant improvement in heart failure symptoms, functional status, and LV function to occur after CABG, the myocardium should have at least 20% viability (6,7).
PET, and SPECT scan), echocardiography (resting and Dobutamine Stress echo), and MRI can be performed to assess the viability and extent of reversibility of the ischemic myocardium. Some studies have suggested that for significant improvement in heart failure symptoms, functional status, and LV function to occur after CABG, the myocardium should have at least 20% viability (6,7).
Positron emission tomography (PET) using perfusion (Rubidium-82 or 13N-ammonia)/metabolic (F-18 deoxyglucose (FDG) or carbon-11 acetate) tracers is considered the “gold standard” method for detecting viability (7). It has been shown that a region showing high FDG uptake relative to myocardial blood flow (perfusion/metabolic mismatch) represents ischemic, stunned, or hibernating myocardium, whereas myocardial scar represents decreased perfusion at rest with corresponding decreased metabolism (perfusion/metabolic match). The average positive and negative predictive accuracy of PET for predicting improved function after revascularization is 82% and 83%, respectively, with an overall predictive accuracy of 82% (8,9,10). The improvement in global LV function and symptomatic improvement is directly related to the number of viable myocardial segments. The presence of perfusion/metabolic mismatch can identify patients at increased risk for future cardiac morbidity and mortality.
Dobutamine stress echocardiography (DSE) can be used to identify viability and helps in differentiating between stunned and hibernating myocardium (11,12,13,14). A uniphasic response indicates myocardial stunning, is denoted by augmentation of segmental wall motion with low doses of dobutamine, and increases with higher doses. The reduction of augmentation with higher doses is indicative of ischemia and hibernation and is termed a biphasic response. DSE has been found to have an overall accuracy of 86%, a specificity of 91%, and sensitivity of 68% for prediction of segmental recovery. It is also predictive of outcome. Bonow (15) et al. illustrated the data from 15 studies, involving 402 patients with ischemic cardiomyopathy. The positive predictive value of DSE to predict recovery after revascularization was 83%, with a negative predictive accuracy of 81%. Cardiac magnetic resonance imaging (MRI) is a clinically valuable alternative to PET in predicting LV functional recovery, following surgical revascularization. It helps in the assessment of tissue perfusion after recanalization, evaluation of myocardial contractile reserve, and characterization of myocardial cellular membrane function (16). Low-dose dobutamine has been combined with MRI for the identification of residual myocardial viability in patients with ventricular dysfunction. The overall accuracy of low-dose dobutamine MRI is 93% compared to 90% by PET (17,18,19). Cine MRI with a Gadolinium-based contrast agent to determine the transmural extent of myocardial viability has been used. New modalities to detect hibernating myocardium include 99mTc-sestamibi, perfusion imaging, nuclear magnetic resonance spectroscopic imaging and ultrasonic tissue characterization.
Revascularization in these high-risk patients should be expeditiously performed. Meticulous myocardial protection and complete revascularization are crucial for good postoperative outcomes.
Several studies have shown that CABG can be performed in high-risk patients with severely impaired LV function (EF < 30%) with low operative mortality, which rivals that of cardiac transplantation. Patients with severe LV dysfunction make a special subset in whom the operative risks are but symptomatic and functional improvement occurs with improved late survival. In the past, perioperative mortality after CABG in patients with EF < 25% has been reported to be between 10% and 37%, but more recent reports indicate mortalities between 2.5% and 8%. At Yale University 188 patients with LVEF < 30% underwent CABG. The mortality in the elective group was 2.8% with 1-, 3-, and 5- year survival of 88%, 77%, and 60% respectively. The functional class improved from 3.1 to 1.4, and LVEF improved from 23.3% to 33.2%.
At the Cleveland Clinic, 1,062 patients underwent primary CABG with or without associated mitral valve surgery and LV reconstruction. The study analyzed the perioperative morbidity, mortality, and late survival. The three groups consisted of CABG (Group I, 728 patients), CABG and mitral valve surgery (Group II, 168 patients), and CABG with LV reconstruction (Group III, 166 patients). The median length of in-hospital stay was 8, 12, and 11 days, respectively. The in-hospital mortality was 2.6%, 3.6%, and 1.2% and overall survival at 1 year, 3 years, and 5 years was 91%, 81%, and 72% in all three groups.
Another recently published prospective study by Shah et al. (20) included 57 patients with a mean age of 67 years and LVEF <35% who underwent CABG. The majority of these patients were in NYHA Functional Class III-IV. The 1-, 5-, and 10-year survival rates were 83%, 56%, and 24%. Short-term and event-free survival was independently and positively correlated with large reversible perfusion defects in preoperative thallium scans.
Ascione and colleagues (21) compared on-pump (n = 176) and off-pump (n = 74) techniques and analyzed the early and midterm outcomes in patients with severe LV dysfunction. The 30 day, 1-year, and 3-year mortality rate was 97%, 92%, and 87% in the on-pump group. In the off-pump group it was 92%, 85%, and 73%, respectively. Although the mortality in the off-pump group was higher than in the on-pump group the differences were not statistically significant.
Improved myocardial protection techniques, use of internal mammary artery, platelet inhibitors, angiotensinconverting enzyme inhibitors, statins, beta-blockers, diuretics, intraaortic balloon pumps, and defibrillators, in appropriate patients have contributed immensely to improved
outcomes following revascularization. Recent evidence from the SOLVD (Studies of Left Ventricular Dysfunction) database shows that of the 5,410 patients with LVEF < 35%, 35% of these patients had previous CABG and the all cause mortality in this group was 26% less at 3 years than the group who had not had bypass surgery. In a selected group of patients with advanced left ventricular dysfunction caused by an ischemic myocardium, CABG may preserve the remaining viable myocardium, provide relief of symptoms, and offer survival of > 60% after 5 years (22).
outcomes following revascularization. Recent evidence from the SOLVD (Studies of Left Ventricular Dysfunction) database shows that of the 5,410 patients with LVEF < 35%, 35% of these patients had previous CABG and the all cause mortality in this group was 26% less at 3 years than the group who had not had bypass surgery. In a selected group of patients with advanced left ventricular dysfunction caused by an ischemic myocardium, CABG may preserve the remaining viable myocardium, provide relief of symptoms, and offer survival of > 60% after 5 years (22).
VALVE SURGERY IN DILATED CARDIOMYOPATHY
Mitral Valve Repair
Functional mitral regurgitation frequently occurs with dilated cardiomyopathy because of annular dilatation. The mitral valve apparatus consists of the annulus, leaflets, chordae tendinae, and papillary muscles. The relationship of these components to one another and with the LV wall is a crucial determinant of left ventricular function. Displacement of the papillary muscles occurs with decreased coaptation of the leaflets with or without restricted leaflet motion. This leads to increased left ventricular volume overload and worsens the LV dilatation and MR. The mitral leaflets remain anatomically normal on echocardiography. In ischemic mitral regurgitation infarction of the papillary muscles may occur and subsequent elongation or rupture may occur. The other mechanism that can cause MR is asynergy of the papillary muscle, or the ventricle that results in mitral regurgitation, located in the commissural area of the same side as the asynergy (23). Recently, real-time, three-dimensional echocardiography (24) has been used to study the geometric differences of the mitral apparatus between ischemic and dilated cardiomyopathy with significant mitral regurgitation. The angle between the annular plane and each leaflet (anterior Aα, posterior Pα) were measured. The pattern of deformation from the medial to the lateral was asymmetrical in ICM-MR and symmetrical in DCM-MR. The clinical implications of these needs to be studied. It is now a well-recognized fact there is increased impetus to perform valve repair with an undersized ring rather than by replacement, although this still largely depends on the skill and experience of the surgeon. The repair may also include an “edge-edge” approximation of the anterior and posterior mitral leaflets in the midportion, creating a double orifice of the mitral valve, thus decreasing regurgitation (25).
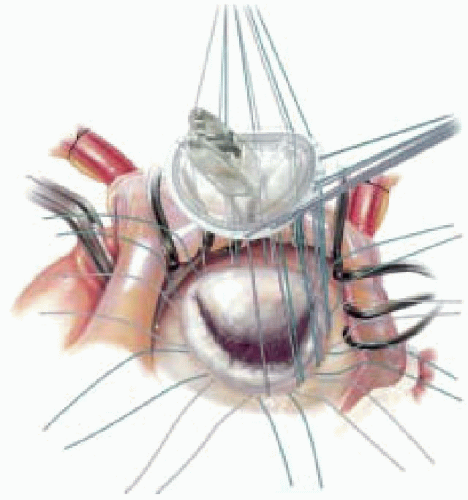
Several groups have now reported the outcomes of mitral repair in patients with severe LV dysfunction. Bolling and colleagues (26,27) studied 150 patients with end-stage cardiomyopathy and severe MR who underwent mitral annuloplasty. The ejection fraction was < 25%, and all patients were in NYHA Class III-IV. Valve repair was performed with an undersized flexible annuloplasty ring (Figs 34.2 and 34.3). The operative mortality was 5% (7 patients), and there were 27 late deaths. Mean follow-up was for 45 months (2-83 months). There was an improvement in both the functional class (Class I-II) and in the ejection fraction. The 1-, 2-, 3-, and 5-year actuarial survival following mitral repairs in patients with severe LV dysfunction was 82%, 71%, 68%, and 51%, respectively.
 FIGURE 34.2. Asymmetric mitral annuloplasty ring (Carpentier-McCarthy-Adams IMR Etlogix ring) for Type IIIb MR. The ring has a reduced AP diameter, dipped P3 region, and reduced P2-P3 curvature. |
At the Cleveland Clinic (28) between 1990 and 1998, 44 patients with mitral regurgitation and LVEF < 35% underwent mitral repair. The 1-, 2-, and 5-year survival rates were 89%, 86%, and 67%, respectively. The average length of in-hospital stay was 9 days. Heart failure and sudden death accounted for 62% of the late deaths. Freedom from readmission for heart failure was 88%, 82%, and 72% at 1, 2, and 5 years.
At the Cleveland Clinic (25) between January 1997 and October 2001, 224 patients with end-stage heart failure underwent mitral repair, using the Alfieri stitch, and 188 had concomitant annuloplasty. Of these 143 patients, XX had ischemic cardiomyopathy, 31 had myxomatous disease, 27 had dilated cardiomyopathy, and 14 had hypertrophic obstructive cardiomyopathy. Preoperative MR was graded between III-IV. The in-hospital mortality was 2%. During the first three months, absence of MR declined to 40%, and prevalence of 3+ MR increased to 14%. Survival at 5 years was 65%. However, results indicate that there is a significant late recurrence of mitral regurgitation in patients with ischemic cardiomyopathy. Fourteen patients (12 within 2 years) required mitral valve replacement and 7 required heart transplantation. The reasons attributed to the failure in this group were progressive annular dilatation, ventricular remodeling, and the use of a flexible ring for the annuloplasty.
Aortic Valve Surgery
Aortic valve disease can manifest with angina, dyspnea, palpitations, syncope, or sudden death. Echocardiographic data should be interpreted with caution in patients with aortic stenosis and low transvalvar gradient, because it is difficult to determine whether there is true aortic stenosis or underlying cardiomyopathy with mild to moderate aortic stenosis. Stress echo and cardiac catheterization help in confirmation of the severity of stenosis. Patients with AR who present with advanced LV dysfunction pose a difficult management problem. Surgery for aortic valve (AV) stenosis with severe LV dysfunction and low transvalvular gradient, aortic regurgitation (AR) with severe LV dysfunction can be performed safely with acceptable results and improved survival.
At the Cleveland Clinic Foundation (29,30) between 1990 and 1998 a group of 68 patients with an AVA < 0.75cm2, LVEF < 35%, and AV gradient < 30mm Hg, who underwent AVR and 89 patients who did not undergo AVR, were evaluated. Propensity analysis was used to compare a cohort of 39 patients in the AVR group and 56 patients in the control group. The 1- and 4-year survival rates in the AVR group were 82% and 78% as compared with patients in the control group with 41% and 15%, respectively. The predictors of survival were AVR, age, and serum-creatinine levels. Between 1985 and 1995, Conolly et al., from the Mayo Clinic, reported their results on 52 patients with a mean age of 71 years, undergoing AVR with AS, LVEF < 35%, and mean transvalvular gradient < 30 mm Hg. Concomitant CABG was performed in 32 patients. The perioperative mortality was 21%, with 10 more deaths during the follow-up period. In this study, advanced age and smaller prosthesis size were significant predictors of hospital mortality by university analysis. An international multimember study by Blackstone et al. (31) quantified the relationship between prosthesis-patient size and long-term survival after AVR. 13,258 patients who had undergone AVR were followed for 15 years. The study showed that prosthesis-patient size down to 1.1 cm2/m2 did not reduce intermediate or long-term survival after AVR. However, prosthesis-patient size under 1.2 cm2 increased the 30-day mortality by 1% to 2%. These results were more pronounced with mechanical valves. Similarly, AV replacement in patients with chronic AR with severe LV dysfunction (32,33,34) has shown improved survival when compared with patients who have undergone AVR before 1990. At the Cleveland Clinic, 34 patients with a mean age of 56.4 years underwent AVR after 1990. Several of these patients were listed for cardiac transplantation. There was no perioperative mortality in this group. The 1-year and 5-year survival rates were 97% and 84%, respectively. These results are quite comparable to the outcomes of patients undergoing AVR with moderately impaired or good LV function. Therefore, an aggressive approach to the management of aortic valve disease with severely impaired left ventricular function is recommended.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree