Surgical Considerations in Aortic Valve Surgery
Cristiano N. Faber
Nicholas G. Smedira
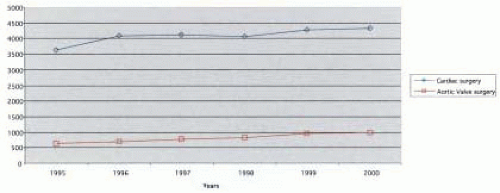
Aortic valve disease is the number one indication for valve replacement. At the Cleveland Clinic Foundation, the number of aortic valve operations has steadily increased over the last five years, with almost 1,000 patients having some type of aortic valve surgery between 1999 and 2000 (Fig. 29.1). With the increasing life expectancy of the American population (1), these numbers will continue to rise with an increasing prevalence of degenerative aortic disease among older patients.
The surgical treatment of aortic valve continues to evolve. The development of new valve prosthesis, surgical techniques, and intraoperative echocardiographic assessment of the anatomy and function of the aortic valve contributed to a more individualized approach to each patient. Intraoperative echocardiography, especially transesophageal echocardiography, offers the surgeon a more detailed and dynamic understanding of the aortic root, valve, and left ventricular outflow tract. In addition to its clinical benefits, routine intraoperative TEE has also been shown to have cost-saving implications in patients undergoing valve surgery (2).
ANATOMY/PATHOLOGY
There are two mechanisms of aortic valve failure: aortic stenosis and aortic insufficiency. A diseased aortic valve can present both abnormalities at the same time but most frequently one pattern will be dominant.
Aortic stenosis (AS) is caused by calcific aortic stenosis (congenital bicuspid valve), rheumatic disease, and atherosclerotic degenerative process. The manifestation of all forms is the same and represents a decrease in the effective valve orifice area and obstruction to the ejection of blood from the left ventricle (LV). This increased afterload
will lead to concentric ventricular hypertrophy and eventually LV dysfunction.
will lead to concentric ventricular hypertrophy and eventually LV dysfunction.
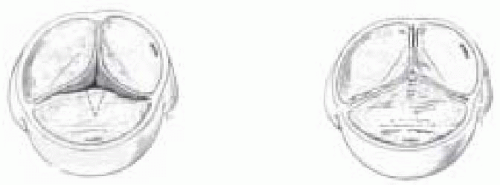
Congenital bicuspid valve abnormalities are characterized by unequal cusp size due to the fusion of right and left cusps, presence of a central raphe, and smooth cusp margins (5). The abnormal flow through the valve is associated with wear and tear of the cusps, which predisposes to calcifications that are more pronounced on the raphe of the conjoined cusp and at the commissures and adjacent aorta (6). Over time, the slow calcification results in significant stenosis during the fifth to sixth decades of life (the most common cause of AS in this age group) (6). Usually the stenosis is accompanied by trivial regurgitation.
Degenerative-calcific process is the most common cause of AS in the adult (7). Histological analysis of the early lesions of valvular aortic stenosis show displacement of the subendothelial elastic lamina; accumulation of a cellular infiltrate of macrophage and T lymphocytes; and deposition of protein, lipid, and calcium. The adjacent fibrosa is also abnormal, with accumulation of fibrous protein, lipid, and calcium minerals (8,9). It is postulated that the development of aortic stenosis is an active process, in part mediated by chronic inflammation, rather than a consequence of aging, and evidence suggests that this inflammatory process is fueled by atherosclerotic risk factors (6).
Apoptosis may also play a role in the mechanism of valvular disease (10). Apoptosis of fibroblasts and endothelial cells of the aortic valve, ranging from focal disruption of individual endothelial cells to extensive denudation of entire endothelium, leads to calcific deposits within the cellular fragments, and this programmed cellular death is increased by hypercholesterolemia (11).
The aortic valve is tricuspid and the calcification involves the cusps, commissures, annulus, sinuses of Valsalva and ascending aorta. It is often present in patients over 65 years of age and its prevalence increases with age. Associated rheumatic disease can accelerate this process, which, despite the low incidence in the United States, is still very prevalent in developing countries.
Aortic insufficiency (AI) can be caused by native valve endocarditis, congenital aortic valve disease, myxomatous aortic valve, rheumatic disease, and ascending aortic enlargement from multiple pathologies (please refer to Chapter 35, Assessment in Surgical Procedures for Congestive Heart Failure, for valve insufficiency associated with ascending aortic/aortic root pathologies). The main mechanism is a lack of coaptation of aortic cusps resulting in retrograde flow into the LV during diastole.
In endocarditis, the insufficiency can be caused by the presence of vegetations, injury of the commissure with prolapse of the cusp, perforation of the cusp, and destruction of the aortic annulus due to an abscess cavity.
AI caused by prolapse of the cusps of the aortic valve is more common in congenital bicuspid aortic valve and is caused by prolapse of the free edge of the conjoined leaflet (12), while in myxomatous degeneration of the aortic valve the prolapse is due to redundant and thickened cusps.
Predominant aortic valve insufficiency is not a common presentation of rheumatic disease, and when it happens it is due to shortening of the cusps caused by scar.
SURGICAL CONSIDERATIONS: AORTIC VALVE REPAIR
When feasible, aortic valve repair is the surgical treatment of choice. In adults, stenotic valves are rarely amenable to repair as decalcification procedures were associated with early failure so that now most efforts at valve repair occur in patients with aortic insufficiency (13,14).
Repair of an insufficient aortic valve requires an understanding of the mechanisms of valve failure. The intraoperative echocardiogram provides the detailed information to help formulate repair plans and assess the results of the repair. Identification of the diseased cusp, mechanism of regurgitation, size of the annulus, and presence of calcification of the aortic annulus are paramount when choosing the repair technique.
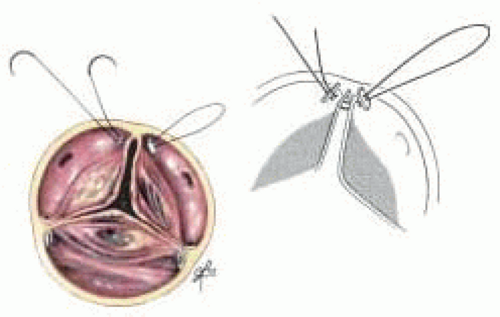
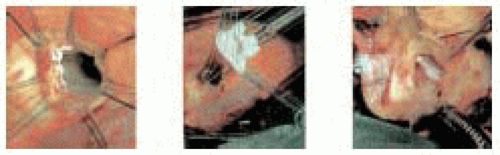
In the insufficient tricuspid aortic valve the main cause of prolapse is elongation of the leaflet with prolapse or rupture of the free edge of the cusp at an area of fenestration. In those cases a triangular resection of the leaflet is performed (Fig. 29.2).
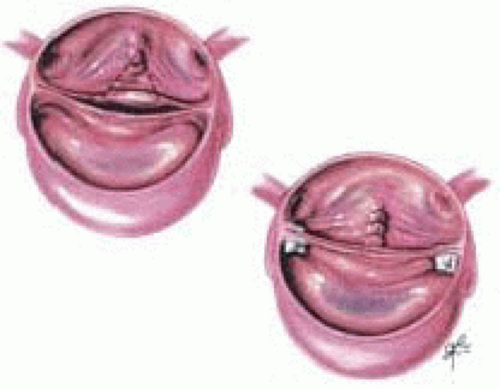
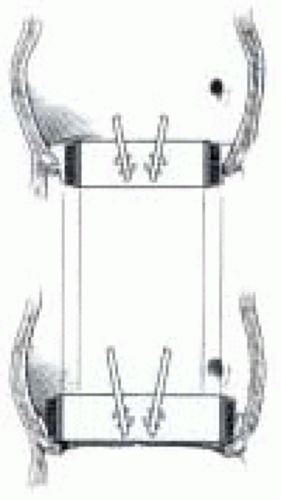
Frequently an annuloplasty needs to be added to the repair to reduce the circumference of the annulus and increase coaptation. This is accomplished by placing a horizontal mattress suture buttressed with Teflon felt at the commissure (Fig. 29.3). The amount of commissural plication is determined by the level of the suture. The lower into the left ventricular outflow tract the suture is the greater the annular plication performed (Fig. 29.4).
For repair of a bicuspid aortic valve the technique is very similar with the resection or plication of the redundant central portion of the prolapsing leaflet, at the site of the raphe, followed by annuloplasty (Fig. 29.5).
Those techniques are shown to effectively eliminate aortic insufficiency (15). To achieve good long-term results, at the end of the repair there should be minimal or no aortic insufficiency.
At the Cleveland Clinic, 94 bicuspid valves and 33 tricuspid valve repairs were analyzed. Tricuspid valve repair, most often involving the right coronary cusp (82% of the time), was less reproducible than bicuspid valve repair. Persistent intraoperative aortic regurgitation requiring valve replacement occurred in 15% of the tricuspid valve repairs versus 0% for bicuspid repairs. Overall freedom from reoperation if the patient left the operating room without AI was 93% for bicuspid repairs and 83% for tricuspid valve repairs at 5 years.
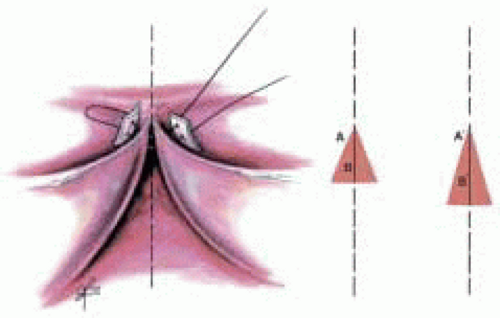 FIGURE 29.4. The amount of annular plication is determined by the level where the suture is placed. The lower the suture is placed the more annular plication is achieved (AB → A’B’). |
SURGICAL CONSIDERATIONS: AORTIC VALVE REPLACEMENT
The implantation technique for a stented prosthesis is the same for mechanical as well biological valves and often consists of placement of non-everting braided polyester mattress sutures, with or without pledgets (Fig. 29.6). The native valve must be excised and the annulus carefully debrided to maintain left ventricular and aortic continuity. The sutures are passed through the sewing ring of the valve and the valve is seated (Fig. 29.7).
Although the technique is the same, for mechanical prosthesis, there are different positions in which the valve can be seated in the aortic annulus, depending on the type of valve utilized. The valve can be implanted in an intraannular or supraannular position depending on its design (Fig. 29.8).
The advantage of the supraannular position is that the valve itself sits above the annulus, because the sewing
cuff is positioned at the inflow level of the valve, moving the housing of the valve to a supraannular position (Fig. 29.9). This theoretically allows one to place a larger valve in the same patient with lower pressure gradients than a smaller valve implanted in the intraannular position (Fig. 29.10).
cuff is positioned at the inflow level of the valve, moving the housing of the valve to a supraannular position (Fig. 29.9). This theoretically allows one to place a larger valve in the same patient with lower pressure gradients than a smaller valve implanted in the intraannular position (Fig. 29.10).
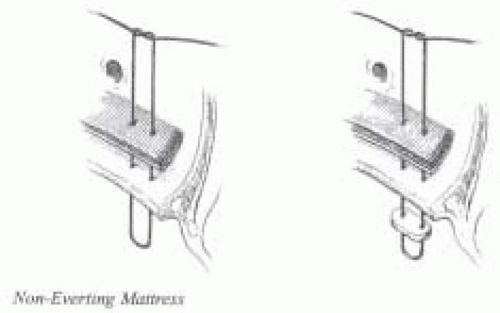 FIGURE 29.6. Most common type of suture technique utilized for implantation of stented valves. (Courtesy of Sulzer CarboMedics, Inc.) |
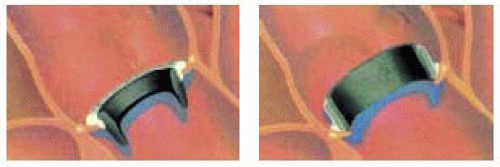 FIGURE 29.9. Position of the aortic valve in relation to the aortic annulus. (Courtesy of Sulzer CarboMedics, Inc.) |
This would be beneficial in cases were the aortic root is small, avoiding the increased morbidity and mortality associated with aortic root enlargement (16). These valves have been used with low mortality and morbidity, but careful attention needs to be taken when sizing this type of valve. Forcing a large prosthesis into the annulus could cause obstruction of the coronaries, dehiscence of the prosthesis, or paravalvular aortic insufficiency (17).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree