Surgical Approaches to Patients with Chronic Congestive Heart Failure
Edwin C. McGee Jr.
Kathleen L. Grady
Patrick M. McCarthy**
* We wish to acknowledge the editorial assistance of Brandi Carr and Linda Flores-Huerta.
Heart failure afflicts almost 5 million Americans and more than 500,000 new cases are diagnosed annually (1). About 300,000 deaths annually are attributed to heart failure as either a primary or contributory cause. With an estimated 1-year mortality of 50%, survival is dismal for medically treated New York Heart Association (NYHA) Class IV, or stage D heart failure patients. Medical treatment with angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and resynchronization therapy are cornerstones of care. Heart failure unresponsive to maximal medical therapy occurs in 60,000 patients each year (2).
Heart transplantation is successful surgical treatment for end-stage heart failure. Due to a limited pool of suitable donors, the number of heart transplants performed annually has been fairly static at just over 2,000 per year in the United States (Fig. 44-1) (3). About 500 to 1,000 patients die each year while on the United Network for Organ Sharing (UNOS) waiting list (4). In addition, end-stage heart failure patients may have comorbid conditions that preclude transplantation, or patients on the list may develop complications that preclude transplantation.
Interest in alternative surgical therapies for patients with advanced heart failure has increased. Some surgical therapies may delay listing for a heart transplant or preclude the need for transplantation altogether. Many individuals with heart failure stemming from ischemic or valvular lesions can potentially be helped by conventional surgical procedures such as coronary revascularization and valve repair or replacement. Furthermore, certain individuals with stage D heart failure are candidates for more advanced surgical therapies such as transplantation and mechanical assistance. Some potential candidates for conventional surgery need mechanical assistance as a backup strategy in case difficulty is encountered during weaning from bypass. The determination of the best treatment for any particular patient is not always straightforward and is best made by a multi-disciplinary team led by cardiologists and surgeons who have specific interest and training in the management of patients with heart failure. In this chapter we will outline the approach we take when thinking of surgical options for patients with heart failure.
Conventional Surgical Procedures
Coronary Artery Disease
Ischemic Cardiomyopathy
Ischemic heart disease remains the leading cause of congestive heart failure (CHF) in the United States and the developed world, accounting for 40% to 70% of cases (5,6). The randomized Coronary Artery Surgery Study (CASS) and VA trials included patients with left ventriclar (LV) dysfunction (7,8). Both demonstrated improved survival for surgical versus medical therapy in patients with coronary artery disease and LV dysfunction. A second randomized VA study of patients with unstable angina demonstrated improved survival for patients with LV dysfunction who underwent surgical revascularization (9). The CASS registry, a nonrandomized portion of the CASS trial, demonstrated a lower rate of sudden cardiac death in patients with LV dysfunction who had been revascularized (10). However, patients with a left ventricular ejection fraction (LVEF) <35% were included in none of these studies, as they were felt to be too high-risk to undergo coronary revascularization.
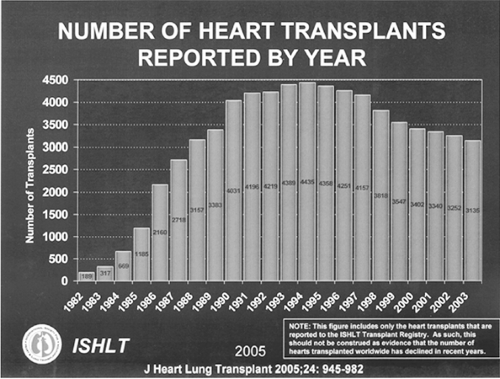 Figure 44-1 Number of heart transplants in the United States by year (International Society of Heart and Lung Transportation). |
Even with modern, state-of-the-art pharmacotherapy, survival is poor for medically treated patients with ischemic cardiomyopathy (11,12,13,14,15,16,17). The Assessment of Treatment with Lisinopril and Survival (ATLAS) trial studied the effect of high- and low-dose lisinopril in 2,035 patients with ischemic cardiomyopathy. The high-dose group had an 8% decrease in mortality after 4 years when compared to the low-dose group, but mortality was still 44% (18). In ATLAS, acute coronary events were the cause of death in 54% of patients. The Studies of Left Ventricular Dysfunction (SOLVD) database examined the effect of enalapril on survival in 5,410 patients with LVEF <35%. In SOLVD, patients who had undergone surgical revascularization within 2 years of study entry had improved survival and a lower rate of sudden cardiac death when compared to patients who had not undergone revascularization (19). Numerous retrospective studies (20), summarized in Table 44-1, have shown that coronary artery bypass in individuals with LVEFs <25% can be accomplished with operative mortality rates ranging from 2% to 8%. Improvement in EF after revascularization has also been demonstrated (20) and is summarized in Table 44-2.
Viability testing is of paramount importance in discriminating which patients with multivessel coronary artery disease and LV dysfunction will benefit from revascularization. A recent meta-analysis of 24 studies examined the impact that myocardial viability has on survival after revascularization for ischemic cardiomyopathy. A clear benefit of revascularization for patients with viability was demonstrated (21). This study involved 3,022 patients with an average LVEF of 32%. Follow-up was 25 months. Revascularized patients with viability had a 3.2% annual mortality rate as compared to a 16% annual mortality rate in patients with viability who did not undergo revascularization. Although the results were not statistically significant, patients without viability who underwent revascularization fared worse than those who were managed medically, with annual mortalities of 7.7% and 6.2%, respectively (21). Magnetic resonance imaging (MRI) is our preferred method for discerning viability. In addition to providing information on viability, MRI also clearly defines anatomy for possible ventricular reconstruction. Positron emission tomography (PET) or other nuclear imaging is reserved for those individuals who cannot undergo MRI because of pacing or automatic internal cardiac defibrillator (AICD) hardware.
LV dysfunction is a risk factor for coronary artery bypass, and individuals with uncompensated heart failure have a very high operative mortality. Although outcomes were better than those in the medical arm, the operative arm of the randomized Should We Revascularize Occluded Coronaries in Cardiovascular Shock (SHOCK) trial had a very high event rate, with 30-day and 1-year mortality rates of 42% and 56%, respectively (22).
Although no randomized trials comparing outcomes of medical and surgical therapy for ischemic cardiomyopathy have been completed, it is apparent that most people with ischemic cardiomyopathy die from ischemia, and much evidence exists to support the beneficial effect of surgery. For individuals with compensated heart failure, revascularization leads to improved long-term survival and can be safely performed in patients with viability despite severely comprised ventricular function (23).
Table 44-1 Results of Coronary Artery Bypass Graft for Severe Left Ventricular Dysfunction | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 44-2 Improvement in Enjection Fraction After Coronary Artery Bypass Graft for Severe Left Ventricular Dysfunction in Recent Series | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Left Ventricular Reconstruction
Surgical reconstruction of a dyskinetic aneurysm or akinetic scar to produce a more efficient ventricle is referred to as left ventricular reconstruction (LVR). For patients with ischemic cardiomyopathy and an area of discrete ventricular scar, combined coronary revascularization and LVR is an attractive option. The first ventricular reconstructions consisted of linear aneurysm repairs that removed the thin-walled scar lateral to the left anterior descending artery. In the 1980s, more complete reconstructions (including the septum) were described by Cooley et al., Dor et al., and Jatene (24,25,26).
Theoretical, mathematical, and observational data exist demonstrating the beneficial effects of LVR. Mathematical modeling predicts that resection of a dyskinetic scar will lead to a net improvement in cardiac function and a reduction in ventricular wall stress (27). After LVR, improvements are seen both in myocardial oxygen consumption and myocardial efficiency which lead to an improvement in the neurohormonal milieu of heart failure (28,29,30,31,32,33).
Indications for Left Ventricular Reconstructive Surgery in Ischemic Cardiomyopathy
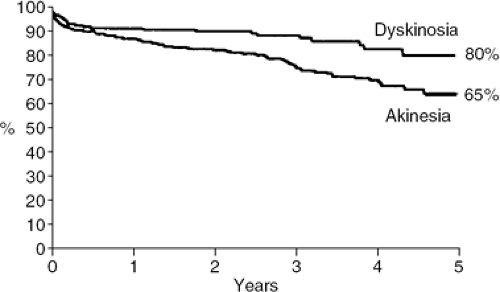
Most patients undergo LVR because of other operative indications, such as left main coronary artery disease, three vessel diseases with positive viability studies, or severe mitral regurgitation. The Reconstructive Endoventricular Surgery returning Torsion Original Radius Elliptical (RESTORE) study is a multicenter registry that looked at the results of 1,198 postinfarction patients who underwent LVR (34). Ninety-five percent of patients underwent concomitant coronary artery bypass surgery (CABG) and 22% underwent concomitant mitral valve repair. Sixty-six percent of patients had LVR or akinetic segment, whereas 34% had LVR for a dyskinetic segment. Thirty-day mortality was 5.3%. Five-year survival was 80% for patients who underwent LVR for a dyskinetic segment versus 65% for those who underwent LVR for an akinetic segment (Fig. 44-2). The average improvement in LVEF was 10%, mean NYHA functional class improved from 3.0 to 1.9, and the 5-year readmission rate for heart failure was 15% (34).
In our practice, reconstruction is performed if there is a discrete thin-walled aneurysm that collapses with venting the left heart. If there is diffuse scar mixed with muscle in all three coronary territories, revascularization without reconstruction is undertaken. We do not reconstruct areas that are thick-walled without visible scar or without scar that is apparent by MRI. As viability studies are only 80% to 90% accurate, we do not reconstruct areas that are, upon direct inspection in the operating room, thick-walled muscle even though preoperative studies indicated nonviable scarred myocardium. If there is any question as to viability, we err on the side of revascularization and forego reconstruction. Most often, reconstruction is performed for a true dyskinetic aneurysm, but we will reconstruct thin akinetic scar.
The Surgical Treatment of Ischemic Heart Failure (STITCH) trial is a multicenter prospective randomized trial designed to study the effect of ventricular reconstruction on survival. Patients with ischemic cardiomyopathy (LVEF <35%) and who are NYHA Class III or IV, with an akinetic or dyskinetic segment are randomized to optimal medical therapy or surgery. The patients in the surgical arm are further randomized to revascularization alone or revascularization plus LVR. The estimated date of study completion is 2008.
Valvular Heart Disease
Mitral Valve
Nearly 50% of patients with an LVEF <35% have 3+ or 4+ mitral regurgitation (MR), and almost 35% have 3+ to 4+ tricuspid regurgitation (TR) (35). Patients with significant mitral regurgitation have been shown to be statistically more likely to die than those without MR following medical therapy (35) post-MI (36) or post-percutaneous coronary intervention (37). Historically, mitral valve surgery in this group of patients with low LVEF was thought to be hazardous and the operative risk prohibitive if the LVEF <40% (38). It was thought that surgical correction of MR in this group removed the so-called pop-off mechanism which allowed the LV to decompress into the low-pressure left atrium. However, this high mortality was more likely related to the state of the art of cardiac surgery in the 1970s and 1980s.
Two mechanisms typically lead to MR in heart failure patients. Annular dilatation (Carpentier type 1) predominates in dilated cardiomyopathy, and posterior leaflet restriction (Carpentier type IIIb) predominates in ischemic cardiomyopathy (39). In advanced ischemic disease, a mixture of the two pathologies often exists. It is important to understand that in both mechanisms the underlying valvular and sub-valvular apparatus appear normal. Bolling et al. (40) were the first to show that mitral valve repair can be performed on patients with severe LV dysfunction and MR with a low operative mortality. Other authors have demonstrated a similar low operative mortality in patients undergoing mitral valve surgery (41,42,43). Intermediate survival, functional class, and ventricular functional changes (improved LVEF, decreased sphericity, and decreased ventricular volumes) have been demonstrated to be improved in patients undergoing mitral valve repair (40,41).
Historically, patients with chronic ischemic mitral regurgitation (IMR) have been dealt with by coronary revascularization alone; many groups still support this strategy (44,45,46). Other groups have shown that even more
moderate amounts of MR in patient undergoing coronary artery bypass do not resolve following coronary bypass alone (47,48) and may reduce survival of patients compared to those without MR (47). Some have also indicated that adding mitral valve repair to this group of patients with 2+ to 3+ MR will improve late survival (49).
moderate amounts of MR in patient undergoing coronary artery bypass do not resolve following coronary bypass alone (47,48) and may reduce survival of patients compared to those without MR (47). Some have also indicated that adding mitral valve repair to this group of patients with 2+ to 3+ MR will improve late survival (49).
Adding mitral valve repair to coronary artery bypass to improve survival and late functional class remains controversial. Some reports have failed to show any benefit (44,45,46) and others have demonstrated that recurrent MR following repair of functional MR secondary to IMR is common (50,51). Three concepts explain the recurrence of IMR. First, work from D. C. Miller’s laboratory has indicated that fixing the septal lateral dimension (AP dimension) is the most important maneuver to effect competence of the valve (52,53). Second, several recent papers indicate that intertrigonal dilatation does occur in patients with ischemic cardiomyopathy (54,55,56,57). Both of these concepts indicate that a complete rigid ring would be preferable to partial bands or suture annuloplasties. Third, several studies confirm the asymmetric nature of IMR with a jet that occurs primarily at the medial commissure (P3 region) (58) and is secondary to tethering of the postero-medial papillary muscle. As such, we believe that the best way to deal with IMR is a complete ring that takes into account the asymmetric dilation at P3.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree