Fig. 23.1
CT scan without contrast showing a retroperitoneal hematoma (RH)
Treatment
Although a widely accepted consensus on the management of patients with RPH does not exist, a reasonable treatment algorithm is shown in Fig. 23.2.
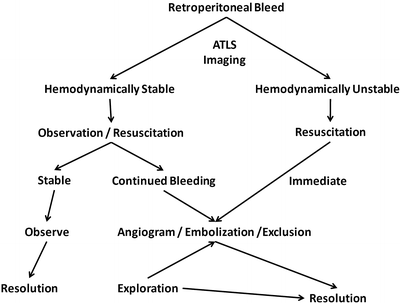

Fig. 23.2
Algorithm for the treatment of a retroperitoneal bleed. ATLS Advanced Trauma Life Support
Conservative Therapy
Hemodynamically stable patients with RPH can be managed with fluid resuscitation, correction of coagulopathy, and blood transfusion. Non-interventional therapy mandates close observation for the development of abdominal compartment syndrome (ACS) which can be fatal if it is not promptly recognized and treated. ACS is defined as sustained intra-abdominal pressure greater than 20 mmHg with end-organ dysfunction manifested as respiratory insufficiency, oliguria, and decreased venous return resulting in severe hypotension.
Endovascular Intervention
Hemodynamic compromise that persists or fails to respond to resuscitation indicates the need for more definitive intervention. Endovascular therapy for RPH requires arteriography and selective vessel catheterization. Selective embolization of a bleeding arterial branch or exclusion of an injured vessel segment with a stent graft can provide definitive hemostasis. Balloon occlusion offers temporary control of the bleeding, while surgical exploration is undertaken. If there is no arterial injury, venography should be considered.
Exploration
Open surgical repair of retroperitoneal bleeding should be reserved for cases when conservative management fails or endovascular measures are either not available or do not control the bleeding. The surgical procedure begins with retroperitoneal exploration and packing. Proximal and distal vascular control is then obtained followed by careful exploration and definitive primary repair or replacement of the injured vessel.
Outcomes
RPH is a potentially deadly condition that carries a mortality rate of 18–60 %. Although there is no level I evidence to guide the management of patients with RPH, it is clear that delayed diagnosis and inappropriate treatment increase the risk of death, and this may account for the wide range in reported mortality rates. Angiography and selective embolization have emerged as effective methods for treating RPH. Several case series document that the technical success of embolization for RPH approaches 100 % resulting in immediate and sustained (greater than 24 h) hemodynamic improvement in more than 90 % of patients. Despite these promising initial results, some patients still require additional hemostatic surgery, and all-cause mortality at 1 month ranges from 32 to 75 % [5]. The ability of embolization to stabilize hemodynamic parameters may depend on the rate of administration of packed red blood cells and fresh frozen plasma (FFP), as well as the systolic blood pressure (SBP) immediately before embolization. Predictors of mortality from RPH include the number of injured sites, SBP before embolization, the need for vasopressive drugs before embolization, and hemodynamic recovery after intervention.
Cervical (Neck) Hemorrhage
Etiology
The most common causes of cervical hemorrhage are traumatic injury, postoperative issues related to specific organs, and the sequelae of therapy for cancer. Projectiles and blades cause most traumatic neck injuries, while postsurgical hemorrhage (including post carotid endarterectomy [CEA] hematoma) represents a common nontraumatic cause of cervical hemorrhage. Penetrating neck trauma represents approximately 5–10 % of all trauma cases that present to the emergency department. About 30 % of these cases are accompanied by an injury outside of the neck zones [6]. In contrast, blunt injury to the carotid and vertebral arteries is rare, occurring in only 1 % of patients with blunt trauma to the face and head. The natural history of blunt neck injuries remains unclear, and the optimal treatment continues to evolve. Biffle et al. proposed a grading scheme for the severity of blunt cerebrovascular injury [7]:
Grade I – intimal irregularity with <25 % narrowing
Grade II – dissection with >25 % luminal narrowing
Grade III – pseudoaneurysm
Grade IV – occlusion
Grade V – transection with extravasation
The ability to accurately describe and classify blunt cerebrovascular injuries will advance research efforts aimed at determining the best treatment strategy.
Carotid blowout syndrome (CBS) is a potentially fatal hemorrhagic complication that occurs in 3–4 % of patients with head and neck cancer [8]. The clinical severity for CBS has been classified into three categories: acute, impending, and threatened [9]. Acute CBS involves complete rupture of the vessel with profuse hemorrhage not controlled with surgical packing. The patient’s condition rapidly deteriorates if immediate resuscitation and stabilization is not achieved before definitive treatment. Impending CBS manifests as short episodes of sentinel hemorrhage that resolve spontaneously or with simple surgical packing. Despite the absence of uncontrollable hemorrhage, complete rupture of the vessel is certain. Threatened CBS describes exposure of the carotid artery as a result of wound breakdown or neoplastic invasion of the carotid system. Although hemorrhage has not yet occurred, rupture is inevitable if the exposed vessel cannot be promptly covered with healthy vascularized tissue.
Presentation
Patients with cervical hemorrhage can present with an external bleeding, an expanding hematoma, or a hemothorax (Fig. 23.3). Approximately 15–20 % of patients are hypotensive, 15–20 % have external hemorrhage, and 30 % have a cervical hematoma [10]. Overt or potential airway compromise may be present due to a combination of direct compression and laryngeal edema. Neurological status should be determined as associated cerebrovascular injury is common and many patients with traumatic cervical bleeding suffer from polytrauma. “Hard” signs that indicate a likely vascular injury include: expanding hematoma, active external hemorrhage from the wound site, a bruit or thrill over the wound, pulse deficit, or distal ischemia presenting as a neurologic deficit.
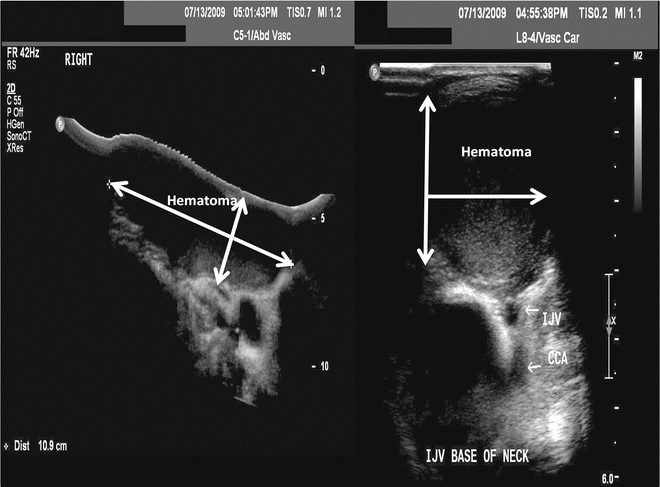

Fig. 23.3
Duplex ultrasound showing a postoperative hematoma in two views (arrow) after line placement. IJV internal jugular vein, CCA common carotid artery
Imaging
For traumatic neck injuries, the ATLS algorithm for imaging based on injury location (zones 1, 2, or 3) should be followed [11]. For an immediate postsurgical bleed, no imaging is required. For a delayed bleed, or hemorrhage after tumor resection (a potential carotid blowout), CT angiography (CTA) and/or catheter-directed angiography is recommended.
Treatment
As with all hemorrhage, the ATLS algorithm should be followed initially as a means of stabilizing and evaluating the patient. Temporary hemostasis can often be obtained with direct manual pressure. For traumatic neck injuries, the general consensus is to repair the common and internal carotid arteries. Ligation usually represents a damage control maneuver in patients with hemodynamic compromise or severe neurological deficits. Figure 23.4 depicts a treatment algorithm for patients with cervical hemorrhage.
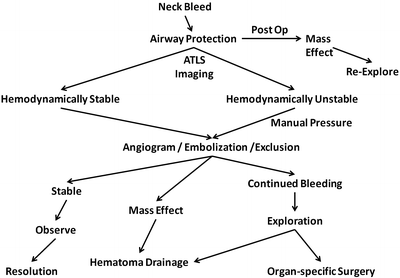

Fig. 23.4
Algorithm for the treatment of a bleed in the neck
Immediate Postoperative Hemorrhage
Patients with cervical bleeding in the immediate postoperative period should undergo surgical reexploration. Intubation should be performed by the most experienced practitioner available under controlled conditions in the operating room. Active bleeding should be surgically repaired while quickly correcting any coagulopathy. Drains should be placed in the wound at the time of surgical reexploration, and intubation should be maintained postoperatively to protect the airway.
Endovascular Intervention
Endovascular therapy usually begins with an aortic arch arteriogram followed by selective catheterization of the arch vessels. Active hemorrhage can be initially controlled with proximal balloon occlusion of the bleeding vessel. Definitive therapy can involve intraluminal placement of a stent graft to cover the injury or open exploration and surgical repair under balloon control. Bleeding from an arterial branch can be treated with selective coil embolization. If carotid occlusion is contemplated, contralateral carotid angiography is required to confirm cerebral cross-filling.
Exploration for Penetrating Trauma
As with all exploration for hemorrhage, proximal vascular control prior to violating the hematoma or exploring the actual injury site is recommended if the anatomy and circumstances allow. If a definitive repair requires prolonged clamping of the internal carotid artery, placing an intraluminal shunt can maintain cerebral perfusion. Key surgical principles are aggressive debridement back to viable vessel wall and the use of autologous tissue to achieve a tension-free repair. The most common surgical repairs involve a vein patch angioplasty or vein interposition bypass graft.
Exploration for Carotid Blowout
Surgical intervention for CBS follows the same principles described above for traumatic cervical injuries, namely, proximal vascular control, intraluminal shunting, and vessel debridement with tension-free repair. In a reoperative surgical field, and in the setting of previous neck radiation, endovascular therapy has gained prominence as a definitive treatment or an adjunctive tool to facilitate surgical repair. As previously described, endovascular intervention can involve intraluminal balloon control, stent graft placement, coil embolization of arterial branches, or permanent carotid occlusion.
Outcomes
The current mortality rate in civilians with penetrating neck injuries ranges from 3 to 6 % [12]. Mortality rates for high velocity penetrating neck trauma have decreased from 11 % in World War I to 7 % in World War II to 3.7 % in the current conflicts [13]. Endovascular procedures for cervical traumas are independently associated with a 35 % reduction in mortality risk [14]. Blunt carotid injury continues to carry a poor prognosis.
Groin Hemorrhage
Etiology
Common causes of groin hemorrhage include iatrogenic injury from arterial catheterization, infection with blowout, and penetrating trauma [15].
Presentation
Patients with groin hemorrhage can present with an external bleeding, an expanding hematoma, or a femoral artery pseudoaneurysm. Some patients present with signs and symptoms of acute limb ischemia, while victims of trauma often have other associated injuries. Wound breakdown or a sinus tract with or without purulent drainage suggests an infectious etiology. These patients may describe a minor bleeding episode – “the sentinel bleed” – which heralds impeding major hemorrhage.
Imaging
For immediate postsurgical bleeding, no imaging is required. Likewise, hemodynamically unstable patients with groin hemorrhage do not require imaging before intervention. For hemodynamically stable patients, imaging choices include duplex ultrasound, CTA, and catheter-directed angiography. The ATLS algorithms provide guidance for imaging traumatic injuries.
Treatment
Active hemorrhage mandates immediate hemostatic maneuvers as delineated in the ATLS algorithm. Direct manual pressure can often provide temporary hemostasis, while arrangements for definitive therapy are made. A treatment algorithm for groin hemorrhage is shown in Fig. 23.5.
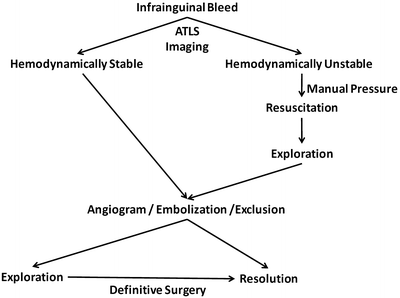

Fig. 23.5
Algorithm for the treatment of a bleed in the groin
Immediate Postoperative Exploration
Postsurgical bleeding should be reexplored under controlled conditions in the operating room. If the case is immediately postoperative, the same proximal site can be used for vascular control. All active bleeding should be addressed and surgically repaired while rapidly correcting coagulopathy. Closed suction wound drainage is recommended postoperatively.
Endovascular Intervention
Angiography of the bleeding groin vessels should be performed via percutaneous access through the contralateral femoral artery. Active hemorrhage can be initially controlled with balloon occlusion of the distal external iliac artery or proximal common femoral artery. Definitive therapy can involve placement of a stent graft or surgical exploration and open repair under balloon control. If arterial branches are bleeding, they can be selectively embolized to achieve hemostasis.
Exploration for Penetrating Trauma
Proximal control for groin hemorrhage may require retroperitoneal exposure of the external iliac artery or isolation of the proximal common femoral artery as it passes deep to the inguinal ligament. If definitive repair requires arterial clamping, shunt placement can be considered but is rarely necessary unless a long delay in reconstituting antegrade flow is anticipated. Concomitant venous injuries may be repaired or ligated depending on the quality of the vessel remaining and the hemodynamic status of the patient. Lower extremity fasciotomies to prevent compartment syndrome should be considered, especially in patients with traumatic injuries. As previously described, surgical principles mandate aggressive debridement of devitalized tissue and the use of autologous tissue for vascular reconstructions. Primary arterial repair can be performed if the defect is less than 2 cm and the edges can be brought together without tension. Vein patch angioplasty or an interposition, reversed vein graft is necessary if primary repair is inadvisable.
Exploration for Femoral Artery/Anastomotic or Pseudoaneurysm Bleeding
Surgical repair of anastomotic or pseudoaneurysm bleeding requires the same techniques previously described for treating penetrating traumatic injuries. The main difference in these clinical scenarios is the possibility of infection which could compromise the long-term integrity of a vascular reconstruction performed within the infected field. If there is evidence or suspicion for infection, an extra-anatomic bypass may be required.
Outcomes
Mortality and morbidity related to groin hemorrhage vary depending on concomitant injuries and preexisting comorbidities. Although a delay in revascularization should be avoided, it is important to note that ischemic time does not correlate precisely with amputation risk nor is it the only factor that determines limb salvage [16].
Arterial Pseudoaneurysm: Diagnosis and Treatment Indications and Options
Etiology
A pseudoaneurysm (PSA) is an arterial rupture in which the bleeding is contained by the surrounding tissues. Blood flow exits through the arterial defect and swirls around within the pseudoaneurysm sac before returning to the artery through the same defect. Unlike a true aneurysm which has all three vessel wall layers, the borders of a PSA do not contain any layers of the arterial wall. PSA is a common complication of percutaneous invasive procedures, occurring in 0.1–0.2 % of diagnostic angiograms and 3.5–5.5 % of interventional procedures [17]. Risk factors for the development of PSA are listed in Table 23.1. Low or high puncture of the common femoral decreases the effectiveness of manual compression resulting in a higher incidence of femoral pseudoaneurysm [18].
Table 23.1
Risk Factors for arterial pseudoaneurysm
Antiplatelet agents (aspirin and clopidogrel) |
Systemic anticoagulation |
Large sheath size (greater than 8 Fr) |
Age greater than 65 years |
Hypertension |
Obesity |
Poor postprocedural compression |
Simultaneous arterial and venous catheterization |
History of peripheral arterial disease |
Hemodialysis |
Complex interventions with multiple sheath exchanges |
Presentation
PSAs commonly present with pain and/or swelling in the groin after catheterization. Swelling from a large PSA or hematoma may lead to compression of nerves and vessels with associated neuropathy, venous thrombosis, claudication, or critical limb ischemia in rare cases. Pressure from the PSA can cause local skin ischemia leading to necrosis and infection. Physical findings associated with PSA include a pulsatile mass, palpable thrill, and audible to-and-fro murmur or systolic bruit. In some cases, none of these physical findings are present, and the patient presents with signs and symptoms of a retroperitoneal hematoma or hemorrhage. These patients have the highest risk for major complications and death.
Imaging
An arterial duplex ultrasound is the imaging modality of choice for diagnosing a femoral PSA with a sensitivity of 94 % and a specificity of 97 % [19] (Fig. 23.6). A comprehensive duplex ultrasound exam should document the size of the PSA, the degree of thrombus (if any) within the PSA cavity, and the communication with the femoral artery including the blood flow velocity pattern within the artery. The common femoral vein should also be evaluated to detect high velocity flow which would suggest an arteriovenous fistula. In many patients, the imaging quality of ultrasound diminishes above the inguinal ligament. Suspicion of a PSA originating from the external iliac artery should prompt evaluation with a CT scan (Fig. 23.7).
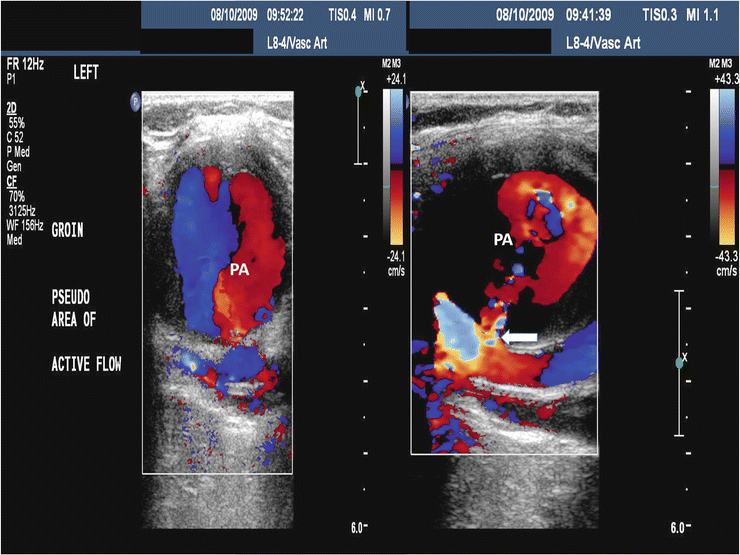
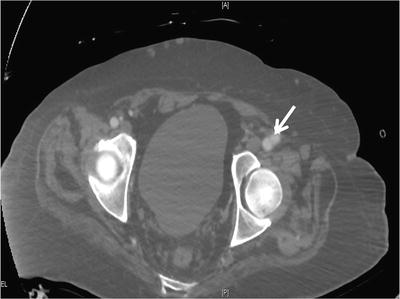

Fig. 23.6
Duplex ultrasound showing a femoral pseudoaneurysm (PA) in two views with the right panel demonstrating the defect in the artery (arrow)

Fig. 23.7
CT scan with contrast showing a common femoral artery pseudoaneurysm (arrow)
Treatment
Small femoral PSAs less than 2 cm in diameter usually clot spontaneously and do not require treatment. Failure to thrombose spontaneously has been associated with concomitant use of anticoagulation or antiplatelet agents [20]. Larger femoral PSAs usually warrant treatment to prevent subsequent complications including rupture or enlargement with compression of adjacent structures. Compression of the femoral vein can result venous thrombosis, while femoral nerve compression can lead to muscle weakness and dysesthesia. A treatment algorithm for femoral PSA is shown in Fig. 23.8.
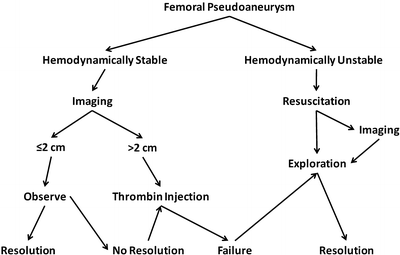

Fig. 23.8




Algorithm for the treatment of an uninfected femoral pseudoaneurysm
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


