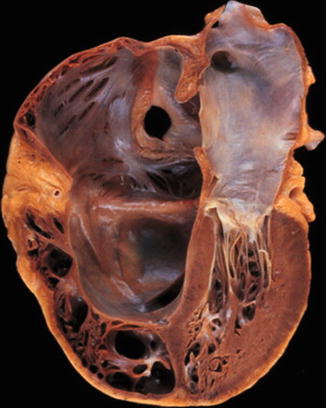
Fig. 10.1
Surgical perspective of the tricuspid valve complex. The tricuspid valve consists of three leaflets: anterior (A), posterior (P) and septal (S). There are two main papillary muscles, anterior (a) and posterior (p). The septal papillary muscle (s) is rudimentary, and chordae tendinae arise directly from the ventricular septum. Relevant adjacent structures include the atrioventricular node (AVN), coronary sinus ostium (CS) and the tendon of Todaro, which form the triangle of Koch. Ao indicates aorta, FO foramen ovale, IVC inferior vena cava, SVC superior vena cava, RAA right atrial appendage, and RV right ventricle (Reprinted with permission from Rogers and Bolling [26])
In Ebstein anomaly, tricuspid valve insertion on the septum and posterior wall is shifted downwards towards the ventricle (Fig. 10.2) [10]. A portion of the ventricular muscle is therefore “atrialised”. All degrees of deformity exist from simple downward shift (by around 1 cm) of relatively normal cusps to a sheet of tissue without obvious commissures low in the ventricle. In extreme cases, the valve cusps do not separate and remain as a diaphragm. Small peripheral apertures then create tricuspid stenosis in contrast with tricuspid atresia. Right bundle branch block is a constant feature of Ebstein anomaly, whilst pre-excitation due to atrial/ventricular continuity is common.
Tricuspid valve stenosis is now rare. In chronic severe rheumatic heart disease, fusion of the three commissures may produce a diaphragm with a fixed central aperture. The valve remains mobile, and significant degrees of fibrosis or calcification are rare. The process does not occur without co-existant aortic or mitral disease. Isolated tricuspid stenosis may occur in the carcinoid syndrome, often in conjunction with pulmonary valve stenosis.
10.2 Tricuspid Valve Regurgitation
The normal tricuspid annulus demonstrates dynamic changes in area and perimeter during the cardiac cycle [11, 12]. The annular dynamics are heterogenous with the anterolateral quadrant showing the most change in area and the anteroseptal quadrant the least. The annular area is greater at end diastole than it is at end systole implying that the orifice of the valve undergoes true expansion with an increase in area during diastole. For anatomical reasons, the annulus towards the free wall of the right ventricle has the greatest degree of flexibility with the anteroseptal quadrant being the most fixed portion [12, 13]. The tricuspid regurgitation encountered during mitral valve surgery is usually due to chronically elevated pulmonary artery pressure and is functional in nature. Around 30 % of those with severe mitral regurgitation have significant tricuspid regurgitation, and 50 % of patients who require mitral valve surgery have tricuspid regurgitation [5, 14]. Chordi from the septal leaflet as well as the septal half of the anterior leaflet attach directly to the septum without papillary muscles. The papillary muscles are attached to the free wall of the right ventricle and to the septum. Changes in the size and geometry of the right ventricle, particularly with increased eccentricity, cause leaflet tethering with reduced coaptation.
In functional tricuspid regurgitation, there is the combination of annular dilatation and leaflet tethering [13]. Progressive right ventricular dilatation leads to annular dilatation and flattening followed by leaflet mal-coaptation [15, 16]. The degree of tricuspid annular remodelling can be used as a surrogate marker of right ventricular dysfunction. It is a progressive process [14–17]. As with the dynamic changes in the normal annulus, annular dilatation occurs predominantly in the anterior and posterior annulus (Fig. 10.3) [12]. The diameter of the anterior annulus may increase up to 40 % and the posterior annulus by 80 %. In contrast, the septal annulus is fixed by its relationship with the fibrous skeleton of the heart. This pulls the anterior and posterior leaflets from their central coaptation zone allowing functional tricuspid regurgitation when the annulus dilates to more than 40 % of its normal size. Approximately 35 % of patients with heart failure have moderate or severe tricuspid regurgitation which is associated with reduced long-term survival [9]. Functional regurgitation in the presence of pulmonary hypertension generally becomes evident when the right ventricular systolic pressure exceeds 55 mmHg. As the degree of regurgitation worsens, the progressive volume overload distends the ventricle and the tricuspid annulus producing more regurgitation. The clinical signs of jugular venous distension, hepatic congestion, ascities and peripheral oedema then follow.
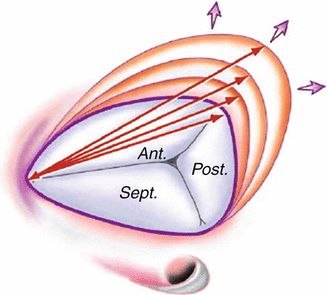

Fig. 10.3
Pathological process of tricuspid annular dilatation. Arrows designate the intercommissural distance that increases with dilatation and that is measured intraoperatively. (Ant. anterior, Post. posterior, Sept. septal) (Reproduced with permission from Dreyfus et al. [18])
Dreyfus et al. described three stages of functional tricuspid regurgitation [12, 18]. Stage 1 is the onset of annular dilatation before valvular regurgitation or with mild regurgitation depending on right ventricular preload, afterload and contractility. Stage II is when annular dilatation becomes significant with persistent tricuspid regurgitation. Failure of leaflet coaptation occurs under all physiological conditions. Stage III is when annular dilatation and right ventricular dilatation result in leaflet tethering. The anterior and posterior leaflets are progressively pulled further apart. Significant tricuspid regurgitation is present in Stage III under all physiological conditions.
It is important to assess tricuspid regurgitation severity taking into account annular diameter, degree of leaflet tethering, degree of right ventricular dysfunction and the pulmonary artery pressure [13, 19]. This is done by echocardiography or cardiac magnetic resonance imaging [20, 21]. Significant tricuspid regurgitation may occur during exercise though trivial at rest. Annulus diameter is measured from the middle of the septal annulus to the middle of the anterior annulus by echocardiography in a four chamber view [20]. It is considered dilated if it is greater than 40 mm or 21 mm/m2 in diastole. This measurement differs from intraoperative surgical measurement of annular diameter between the anteroseptal commissure to the anteroposterior commissure. The latter is the maximal tricuspid annulus diameter in a fully relaxed heart. Normal is around 35 mm and it is considered significantly dilated when >70 mm. This dimension is present in up to half of patients undergoing mitral valve surgery. Intraoperative transoesophageal echocardiography can be used to obtain this dimension by the trans-gastric view. The degree of tricuspid leaflet tethering can be assessed by measuring the distance between the coaptation level of the anterior and septal leaflets and the plane of the tricuspid annulus. This is the tethering height which is significant when >8 mm.
Left-sided valvular heart disease is the most common cause of functional tricuspid regurgitation [14, 22]. Rheumatic, degenerative, and carcinoid diseases are far less common. In the presence of established tricuspid annular dilatation and leaflet tethering, correction of the left-sided lesion and lowering of pulmonary artery pressure may not resolve the tricuspid regurgitation. Dreyfus et al. showed 34 % of isolated mitral valve repair patients to develop significant late tricuspid regurgitation with continued impairment of functional capacity [12, 18]. Calafiore et al. showed tricuspid regurgitation to progress in 40 % of patients after mitral surgery without tricuspid annuloplasty, and this was associated with worse survival and functional capacity [5, 23]. Persistent atrial fibrillation increases the likelihood of progressive tricuspid regurgitation [24]. In contrast, tricuspid regurgitation will not progress following successful mitral surgery if there is no annular dilatation, leaflet tethering or dysrhythmia [22].
10.3 Indications for Tricuspid Valve Surgery
Primary tricuspid pathology occurs in only 8–10 % of patients who need surgery. Secondary tricuspid regurgitation is the most frequent indication [25, 26]. As for mitral regurgitation, tricuspid regurgitation itself leads to right ventricular dilatation and dysfunction, right atrial enlargement and then more annular dilatation and leaflet tethering [22]. As the right ventricle dilates and fails, increased diastolic pressure causes shift of the intraventricular septum towards the left ventricle. Ventricular interdependence may reduce the left ventricular cavity size by compression causing restricted filling and increased left ventricular diastolic and pulmonary artery pressures [27]. The onset of atrial fibrillation may suddenly precipitate symptoms of breathlessness and fatigue and the sequelae of venous congestion. Severe tricuspid regurgitation is a strong predictor of overall mortality independent of age, left ventricular function or pulmonary artery pressure. Equally secondary tricuspid regurgitation complicates the post-operative course of mitral valve surgery and is associated with reduced post-operative survival [14]. Neuhold et al. showed tricuspid regurgitation to be associated with reduced survival in patients with mild, moderate or severe left ventricular dysfunction [7]. In patients with mild to moderate left ventricular dysfunction, tricuspid regurgitation was an independent predictor of negative outcome. As a result of these and other studies, the European Society of Cardiology (ESC) guidelines recommend tricuspid valve annuloplasty for patients with severe secondary tricuspid regurgitation undergoing left-sided heart valve surgery (Class I) [3]. They suggest consideration of intervention in those with moderate secondary regurgitation undergoing left-sided heart valve surgery (Class IIa) when the tricuspid annulus is dilated (>40 mm or 22 mm/m2 on echocardiography). The 2014 American College of Cardiology/American Heart Association guidelines for the management of patients with valvular heart disease recommend intervention for those with severe tricuspid regurgitation under left-sided valve surgery but emphasise that mild/moderate functional regurgitation will progress in 25 % [2]. As a result, repair is advisable when annulus diameter exceeds 40 mm on preoperative echocardiography or 70 mm on direct intraoperative measurement. Others now advocate tricuspid annuloplasty at the time of left-sided heart valve surgery for all significant tricuspid regurgitation regardless of the degree of left ventricular function [5, 6, 18]. Though the natural history of secondary tricuspid regurgitation remains poorly understood, there is sufficient to indicate that it may lead to irreversible right ventricular dysfunction, heart failure and death [4]. King et al. showed that 66 % of patients returning for tricuspid valve surgery late after mitral valve replacement had only mild tricuspid valve insufficiency at the time of the initial operation [24]. The progression was related to persistent pulmonary hypertension and secondary right ventricular cardiomyopathy. Thus, the important issue is not the degree of tricuspid regurgitation but the severity of right ventricular dysfunction. This is determined by preoperative transthoracic echocardiography (tricuspid annular plane systolic excursion >16 mm, annular velocity >10 cm/s and right ventricular end-systolic area <20 cm2).
10.4 Primary Tricuspid Valve Disease
Rheumatic tricuspid valve disease occurs only in association with involvement of the mitral and aortic valves [28]. The rheumatic process results in an incompetent valve with variable degrees of stenosis though in rare cases there may be pure stenosis. All commissures are usually equally fused, but occasionally, fusion is limited to the anteroseptal commissure. Chordal thickening and shortening are usually mild and calcification absent. When stenotic, the orifice of the tricuspid valve is larger than that of the stenotic mitral orifice. The heamodynamic effects of anatomically moderate tricuspid stenosis are the equivalent of tight mitral stenosis.
Isolated acute tricuspid valve endocarditis is rare and usually associated with the habitual intravenous self-administration of narcotic drugs [29]. The organism most commonly involved is Pseudomonas aeruginosa and the second commonest Staphylococcus aureus. A variety of gram-negative bacilli may be involved. Rarely, Candida albicans is the infective agent. The organisms may form masses on the valve leaflets or simply erode and destroy large portions of leaflets and chordi. The classic signs and symptoms of gross tricuspid regurgitation develop though pulmonary symptoms and signs secondary to septic pulmonary emboli may predominate.
Carcinoid tumours originate from Kulchitsky cells in the gastrointestinal tract which produce serotonin [30]. Whilst serotonin is inactivated by the liver, metastatic carcinoid tumour within the liver secretes serotonin into the right heart and pulmonary circulation. The carcinoid syndrome may be produced with bronchospasm, diarrhoea, nausea, malabsorption, flushing and telangiectasia. Some of these patients develop tricuspid and pulmonary valve stenosis through fusion of the commissures, leaflet thickening and chordal shortening. Microscopically, a layer of loose fibrous tissue can often be seen on both surfaces of the tricuspid leaflets. Both tricuspid and pulmonary valve replacement may be required [31].
Tricuspid regurgitation is an uncommon result of severe blunt chest injury in association with right ventricular trauma and rupture of a tricuspid papillary muscle [32]. Usually the anterior leaflet becomes flail or detached from the annulus. Rarely the ventricular septum may rupture. The diagnosis is easily established by the presence of a murmur, signs of raised venous pressure and a history of trauma. Right to left shunting may occur acutely across a patent foramen ovale leading to the mistaken diagnosis of Ebstein anomaly.
Right ventricular dysfunction and tricuspid regurgitation from coronary artery disease is usually the result of extension of an infero-posterior transmural infarction. Given that the blood supply to the right ventricle is most commonly derived from the right coronary artery and acute marginal branches, an inferior myocardial infarction from proximal right coronary occlusion can lead to right ventricular dilatation. Tricuspid regurgitation can also occur with an infero-septal infarction because some tricuspid chordi are directly attached to the interventricular septum. True ischaemic tricuspid regurgitation is usually responsive to coronary revascularisation. Irrespective of infarction, the right ventricle is generally resistant to permanent ischaemic dysfunction and frequently recovers.
10.5 Surgery for Tricuspid Regurgitation
Valve repair is preferable to replacement and is usually possible for functional regurgitation [33]. Tricuspid annuloplasty aims to achieve leaflet coaptation by restoring annulus size and geometry [34]. In patients who require aortic or mitral valve surgery, it is best to determine the need for tricuspid repair preoperatively by transthoracic echocardiography or magnetic resonance imaging. Tricuspid regurgitation can be reduced by general anaesthesia which induces systemic vasodilatation and significant afterload reduction. Reduced preload through veno-dilatation, fasting or impaired right ventricular function attenuate tricuspid regurgitation on intraoperative transoesophageal echocardiography. Even trivial tricuspid regurgitation at the time of mitral surgery can progress in the presence of atrial fibrillation, pulmonary hypertension, annulus dilatation and leaflet tethering [22, 24]. Carpentier, Dreyfuss and others have each demonstrated that concomitant tricuspid annuloplasty can be performed reproducibly without incremental risk [11, 25]. The objective is to achieve a large surface of leaflet coaptation by restoring normal annulus size and geometry. However, in the presence of leaflet, tethering annuloplasty alone may not fully restore competence. Anterior leaflet augmentation may be required to enlarge the surface of coarctation [25, 35].
The heart is approached through a standard median sternotomy. Inspection of the cardiac morphology then helps to determine the approach to mitral and tricuspid valves. The superior and inferior vena cava are circumvented to improve access for cannulation and passage of caval snares. The purse string for the inferior caval cannula is positioned posteriorly at the cavo-atrial junction. Superior vena caval cannulation depends on size of the left atrium. When the left atrium is not enlarged, a right-angled cannula is placed directly into the superior vena cava. This allows access to both right and left atrium through a supero-septal incision. This is carried obliquely from the free wall of the right atrium, across the right atrial appendage and down onto the roof of the left atrium. An incision beginning in the fossa ovalis and continued cranially towards the roof of the left atrium completes the supero-septal approach. This allows unrestricted access to both the mitral and tricuspid valves. One disadvantage of this incision is that the right coronary artery branch to the sino-atrial node is transected which may result in temporary dysrhythmia. If the right atrium and inter-atrial septum are enlarged, a trans-septal incision alone will prove sufficient without opening the roof of the left atrium. If the left atrium is substantially dilated, this can be approached via the inter-atrial groove. A separate right atrial incision is then used to address the tricuspid valve.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree