49 Surgery for Peripheral Vascular Diseases
Etiology and Pathogenesis
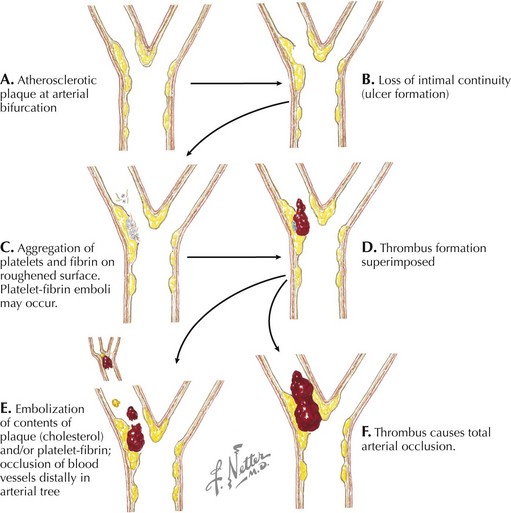
For many years the etiology of aneurysmal disease was believed to be primarily related to atherosclerosis, largely because aneurysmal disease occurs predominantly in elderly hypertensive individuals and is associated with tobacco use. The etiology is now thought to be multifactorial. Genetic predisposition may be involved in up to one third of patients with aneurysms. Microscopic analysis indicates that deficiencies in elastin, collagen, or both may be crucial factors. Collagen-degrading matrix metalloproteinases are probable culprits in aneurysm formation, and current research focuses on their role in the pathogenesis of aneurysmal disease. Elastin and collagen breakdown, which may be accelerated based on a genetic predisposition to produce matrix metalloproteinases, may precipitate an inflammatory reaction. This inflammatory reaction can then contribute to weakening of the arterial wall and eventual dilatation. The presence of several cytokines and systemic biomarkers has been shown to correlate with the presence and size of abdominal aortic aneurysms, and it is likely that a causative relationship exists. Embolic disease, thrombosis, or trauma may also cause arterial occlusion (Fig. 49-1). However, the most common cause of lower extremity arterial occlusion is atherosclerosis. The etiology and pathogenesis of atherosclerosis are discussed in Chapter 44.
Abdominal Aortic Aneurysms
Management and Therapy
Optimum Treatment
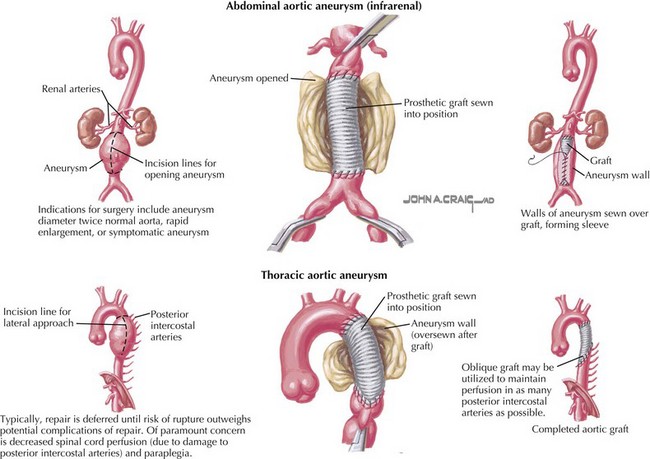
During the past 50 years the surgical technique for AAA repair has remained essentially unchanged, with outcome improvements resulting from advances in preoperative screening and risk stratification, improved anesthetic practice, and intensive care management. Notably, however, the use of more durable synthetic grafts in recent years (rather than using homografts for AAA repair; Fig. 49-2, upper) has also contributed to the improved long-term outcome following AAA repair. Aneurysmorrhaphy involves mobilization and exposure of the aneurysm and the normal artery above and below the diseased section. Blood flow through the artery is arrested for inline replacement of the diseased artery with an artificial one, resulting in major cardiovascular stress during the procedure and for several days after. The combination of cardiovascular stress and the advanced age and comorbid conditions of the patient increases the procedure-associated morbidity and mortality rates. Patients usually require 7 to 10 days of hospitalization and 6 to 8 weeks to recover. However, once patients fully recover from the procedure, long-term follow-up indicates that few patients need further intervention. When further intervention is needed, generally it is because progression of the disease has occurred in adjacent arteries. Ongoing research focuses on the identification of mechanisms to arrest the disease process to prevent spread and inhibit the inflammatory process.
Thoracic Aneurysms
Management and Therapy
Optimum Treatment
As is the case for surgical repair of infrarenal disease, surgical repair of thoracic aneurysms usually requires replacement of the diseased artery. However, the risks associated with surgical repair of thoracic and thoracoabdominal aneurysms are significantly higher than those of AAA repair. One major risk associated with thoracoabdominal aneurysm repair is paraplegia, because perfusion to the spinal cord must be interrupted during the repair. Several approaches have been developed to limit the amount of ischemia, including the use of barbiturates, hypothermia, and spinal cord drainage to increase perfusion pressure via collaterals. Even with these protective approaches, for extensive aneurysms involving the area from the left subclavian artery to the aortic bifurcation, the risk of paraplegia is as high as 25%. For small aneurysms involving a short section of the aorta, the risk of paraplegia is not negligible (2% to 8%). Because of the high risk, treatment is delayed until the risk of rupture is greater than the risk of repair, typically when an aneurysm is 6 cm in diameter (Fig. 49-2, lower). Individuals with Marfan’s disease or other collagen vascular diseases represent an important subset in whom the risk of dissection and/or rupture is increased even at smaller aneurysm diameters, requiring earlier surgical intervention.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree