Chapter 12 Surgery for adults
INTRODUCTION
Recent advances in both surgical and pain management, the evolution of new forms of postoperative physiotherapy support and a reduction in the incidence of clinically significant PPC have provided the stimulus for a re-evaluation of the place of physiotherapy in surgery. The incidence of PPC has shown a gradual reduction over time in these patient populations. In part this is due to a change in the method for the measurement of PPC, and also because anaesthetic and surgical techniques have improved. The use of epidural and patient-controlled analgesic techniques on the ward has also had a profound impact on the incidence of PPC. The incidence of PPC is stated to be 5–10% (Brooks-Brunn 1995), whereas earlier it was reported to be 20–30% (King 1933, Wightman 1968). The introduction of ‘fast track’ postoperative management and minimally invasive abdominal, thoracic and cardiac surgery has also impacted on the physiotherapy management in these patient populations, although more research is needed in these areas.
This chapter outlines the effects of the surgical process, pain and pain management on the respiratory system, the development of PPC, evidence for the physiotherapy treatment of different surgical populations, use of a variety of physiotherapy treatment techniques, management of surgical drips and drains, including underwater seal drainage and brief notes on different types of surgical procedures and lung cancer. The chapter has been written to aid both undergraduate physiotherapy students and cardiorespiratory clinicians in better understanding the area of physiotherapy and surgery. The evidence base underpinning the place of physiotherapy in surgery has been reviewed by Denehy & Browning (2007).
THE SURGICAL PROCESS
General anaesthesia
General anaesthesia (GA) provides the patient with sleep, amnesia and analgesia. Constant monitoring of the patient’s vital signs allow these to be kept within physiological limits. General anaesthesia can be divided into three different stages: induction, maintenance and reversal or emergence. Before induction an intravenous (IV) administration of a combination of an anxiolytic drug with amnesic power such as midazolam, together with a narcotic such as fentanyl, is often given. The narcotic given preoperatively helps to prevent nerve impulses, arising from intraoperative events, from sensitizing central neuronal structures and is called pre-emptive analgesia (Katz 1993). There is some evidence that analgesia given before the painful stimulus reduces subsequent pain, but this remains controversial.
Induction of anaesthesia:
Anaesthesia is usually achieved by IV administration of a short-acting, coma-inducing drug such as propofol or thiopental. Intubation may be performed if the surgery requires administration of muscle relaxants to cause paralysis (as is the case in major surgical procedures such as abdominal surgery). Maintenance of anaesthesia is achieved using inhalational agents such as sevoflurane with nitrous oxide or air with a suitably high inspired oxygen concentration (FiO2). Total intravenous anaesthesia using propofol may be used for maintenance and instead of an inhalation agent. During maintenance, muscle relaxants are often used to aid the surgical procedure and narcotics given for both intraoperative and postoperative analgesia. The process of reversal begins well before the surgeon has finished. Inspired anaesthetic concentrations are scaled back and drugs to reverse paralysis such as neostigmine are given. Analgesia is provided using narcotics or regional analgesia and extubation occurs once the patient can protect their airway (gag reflex) (Euliano & Gravenstein 2004).
Management of acute postoperative pain
It has been suggested that pain in the early postoperative period may be the most important factor responsible for ineffective ventilation, poor cough, impaired ability to breath deeply and sigh, atelectasis, hypoxaemia and respiratory distress postoperatively (Sabanathan et al 1999). It is clear that pain is an important factor that can be modified postoperatively to attenuate some of the above physiological changes associated with surgery. For this reason it is critically important that postoperative pain management is optimum. Inadequate analgesia may delay discharge from hospital, cause sleep disturbances and limit early mobilization.
Reduction in acute postoperative pain facilitates improved patient comfort and satisfaction, reduced length of hospital stay and rehabilitation. Acute postoperative pain is the result of local tissue damage with release of algesic substances (prostaglandins, histamine, serotonin, bradykinin) and generation of noxious stimuli, which are transduced by nociceptors and transmitted by A-delta and C nerve fibres to the neuraxis. Complex modulating influences occur in the spinal cord, producing segmental responses including increased sympathetic stimulation, muscle spasm and increased gastrointestinal tone. Other impulses are transmitted to higher centres via the spinothalamic and spinoreticular tracts producing cortical and suprasegmental responses. These result in further increased sympathetic tone increasing metabolism and oxygen consumption (Ready 1985). The major anatomical targets for relief of postoperative pain are the peripheral tissues, nerve axons in peripheral nerves and dorsal nerve roots, the dorsal horn of the spinal cord and the brain. Many different methods of pain relief, directed to these different anatomical sites are available to patients. Several other factors may modify postoperative pain: these include the site and duration of surgery and the extent of the incision and surgical trauma. However, the physiological and psychological makeup of the patient and past pain experience also play a part. Postoperative pain is often accompanied by changes in autonomic activity, which are sympathetically mediated and include hypertension, tachycardia, sweating and decreased gut motility (National Health and Medical Research Council 2005).
Drug management of postoperative pain
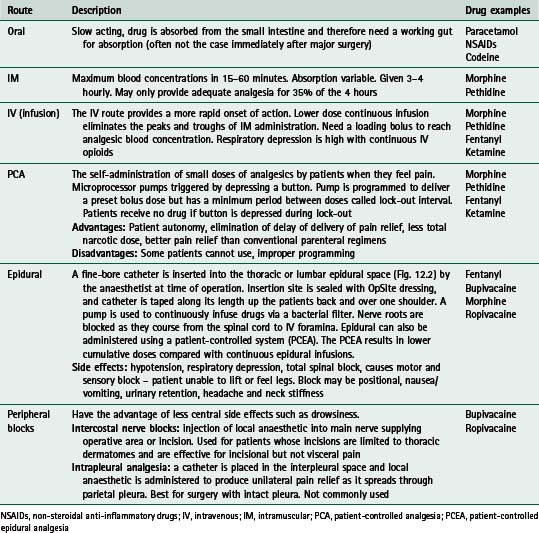
Many different methods of pain relief are available, using several different routes of administration. Opiates and derivatives (such as morphine, pethidine, fentanyl) make up a large proportion of these drugs and morphine arguably remains the benchmark drug (Barrat 1997). Table 12.1 gives a summary of common routes of administration of drugs. In Table 12.2 a list of the common drugs used for postoperative pain is shown, together with their potential side effects.
Table 12.1 Common routes of drug administration for postoperative analgesia
| Route | Description | Drug examples |
|---|---|---|
| Oral | Slow acting, drug is absorbed from the small intestine and therefore need a working gut for absorption (often not the case immediately after major surgery) | Paracetamol NSAIDs Codeine |
| IM | Maximum blood concentrations in 15–60 minutes. Absorption variable. Given 3–4 hourly. May only provide adequate analgesia for 35% of the 4 hours | Morphine Pethidine |
| IV (infusion) | The IV route provides a more rapid onset of action. Lower dose continuous infusion eliminates the peaks and troughs of IM administration. Need a loading bolus to reach analgesic blood concentration. Respiratory depression is high with continuous IV opioids | Morphine Pethidine Fentanyl Ketamine |
| PCA | Microprocessor pumps triggered by depressing a button. Pump is programmed to deliver a preset bolus dose but has a minimum period between doses called lock-out interval. Patients receive no drug if button is depressed during lock-out Advantages: Patient autonomy, elimination of delay of delivery of pain relief, less total narcotic dose, better pain relief than conventional parenteral regimens | Morphine Pethidine Fentanyl Ketamine |
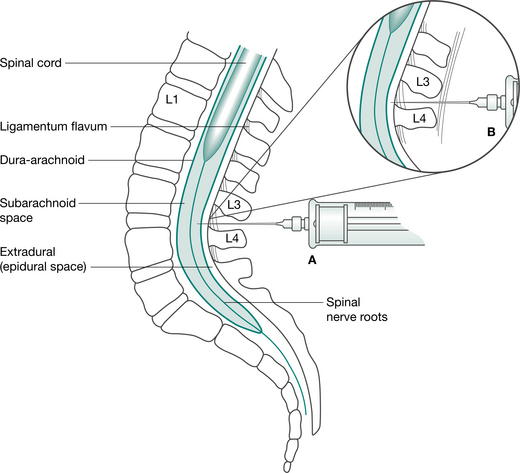
| Epidural | A fine-bore catheter is inserted into the thoracic or lumbar epidural space (Fig. 12.2) by the anaesthetist at time of operation. Insertion site is sealed with OpSite dressing, and catheter is taped along its length up the patients back and over one shoulder. A pump is used to continuously infuse drugs via a bacterial filter. Nerve roots are blocked as they course from the spinal cord to IV foramina. Epidural can also be administered using a patient-controlled system (PCEA). The PCEA results in lower cumulative doses compared with continuous epidural infusions. | Fentanyl Bupivacaine Morphine Ropivacaine |
| Peripheral blocks | Intercostal nerve blocks: injection of local anaesthetic into main nerve supplying operative area or incision. Used for patients whose incisions are limited to thoracic dermatomes and are effective for incisional but not visceral pain | Bupivacaine Ropivacaine |
NSAIDs, non-steroidal anti-inflammatory drugs; IV, intravenous; IM, intramuscular; PCA, patient-controlled analgesia; PCEA, patient-controlled epidural analgesia
Opioids are drugs that bind with specific opioid receptors. They mimic endogenous peptide transmitters involved in pain modulation and act principally within the central nervous system. Non-steroidal antiinflammatory drugs (NSAIDs), such as indomethacin, are also used in management of acute postoperative pain and can act as opioid sparing agents. They decrease production of prostaglandins that sensitize nociceptor nerve endings to inflammatory mediators and also have an antipyretic effect (reduce fever). Local anaesthetic agents such as bupivacaine, block the initiation and propagation of action potentials by blocking sodium channels. They are used to produce nerve root blocks and to depress action potentials in sensory neurons, thereby reducing pain (NHMRC 2005).
The use of multimodality analgesia rather than single analgesic administration is more common in the new millennium as is the existence of hospital pain management teams led by anaesthetists and nurses.
Opiates have significant respiratory depression effects (Sabanathan et al 1999) and they are only partially effective in relieving pain. Richardson and Sabanathan (1997) report that since opioids may only relieve pain transmitted by C fibres, where opioid receptors exist, the sharp pain transmitted by A fibres still exists. More recently, use of local anaesthetics (bupivacaine, ropivacaine) by the epidural route has gained more favour in postoperative pain management, often used in combination with opioids. The introduction of these multimodal methods has also seen an increase in the use of NSAIDs such as indometacin. These are used as opioid sparing agents, but have also been shown to improve analgesia by a reduction in inflammation and stress inhibition (Richardson & Sabanathan 1997). There is level 1 evidence that oral administration of NSAIDs is as effective as intravenous (National Health and Medical Research Council 2005). Oral paracetamol or narcotic drugs may also be added to these regimens. Multimodal or balanced analgesia has been developed in response to the commonly associated side effects of monotherapy – nausea, vomiting, paralytic ileus and respiratory depression in the case of opioids and urinary retention, motor block and hypotension in the case of local anaesthetic agents. Balanced analgesia was also developed to allow early postoperative ambulation and enteral feeding, which minimize the respiratory and gut complications associated with use of opioids, leading to earlier patient discharge from hospital. The drugs are used in smaller doses than if used separately and provide effective pain relief as a result of their synergistic actions (Peeters-Asdourian & Gupta 1999).
The most common methods for pain control following abdominal surgery are patient-controlled analgesia (PCA) with intravenous opioids (Fig. 12.1) and continuous epidural analgesia delivering a combination of local anaesthetic and opioids (Fig. 12.2). In a recent systematic review it was concluded that continuous epidural analgesia is superior to PCA in relieving postoperative pain for up to 72 hours in patients undergoing intra-abdominal surgery. Similarly a large review of analgesia reports that all techniques of epidural analgesia provide better postoperative pain relief compared with parenteral opioid administration (NHMRC 2005). However, PCA remains a common method of analgesic delivery, as patients often prefer it (Werawatganon & Charuluxanun 2005). Continuous infusion may be associated with increased risk of respiratory depression compared with bolus IV administration and evidence for improved pain relief with continuous administration is lacking (NHMRC 2005).
Non-pharmacological methods for managing postoperative pain are generally perceived to be adjuncts to pharmacological methods, but there is growing evidence for the value of their contribution (National Health and Medical Research Council 2005). Education including providing procedural information about treatment (such as provided by physiotherapists preoperatively), combined with sensory information describing the sensory experiences a patient may expect and information regarding coping strategies, may be effective in reducing negative affect, pain medication use and improving clinical recovery after surgery (National Health and Medical Research Council 2005). Preoperative education may encourage a more positive attitude toward pain relief, although there is no evidence that preoperative education about pain has any effect on postoperative pain after cardiac surgery (National Health and Medical Research Council 2005). Implementation of an acute pain management service may also improve pain relief.
Measurement of pain
Pain is difficult to measure as it is a purely individual and sensory experience (Dodson 1985). Postoperative pain is acute and initiated by tissue injury during surgery, but reduces with time and the natural healing process. The measurement of pain is often necessary to assess the results of an intervention or to measure intensity of pain, such as postoperative pain. Regular measurement of pain leads to improved acute pain management. Most measures of pain are based upon self-report but can provide sensitive and consistent results if performed properly (National Health and Medical Research Council 2005).
Several different instruments may be used to measure pain, these include:
Except for the McGill pain questionnaire, these methods are unidimensional; that is, they only measure intensity of pain in absolute terms or changes in pain intensity. Verbal scales may use words that have different meanings for different people, such as mild, moderate or severe pain. These categorical scales are quick and simple but less sensitive than numerical rating scales such as the visual analogue scale (VAS) (National Health and Medical Research Council 2005). The McGill questionnaire uses 20 groups of two to six words and the patient is asked which word in each group best describes their pain. While this offers more valid information than VDS, it is very time consuming and it not used extensively for the measurement of acute postoperative pain. Verbal rating scales are commonly used in clinical practice and use of the VAS is the most commonly used method. Visual analogue scales usually consist of a straight line, 10 cm long, the extremes of which are taken to represent the limits of the subjective experience being measured. In the case of pain measurement, one end of the line may be defined as ‘no pain’ and the other as ‘severe pain’ or ‘worst possible pain’. The subject is asked to place a mark on the line corresponding to the severity of their pain. The distance from the mark to the end of the scale is taken to represent pain severity. The most common way to use a VAS in the study of postoperative pain is to ask the patient to score the pain they are experiencing at the time of completion of the VAS. Visual analogue scales may also be used to obtain a pain score that reflects pain or pain relief over the preceding 24 hours. Commonly, physiotherapists ask patients to rate their pain on activity in the postoperative period to provide more meaningful information. The VAS has been shown to be a linear scale for patients with postoperative pain of mild to moderate intensity. Therefore results are equally distributed across the scale so that the difference between each number on the scale is equal. It is reported that values greater than 70 mm are indicative of severe pain while values between 45 and 74 mm represent moderate pain and those between 5 and 44 mm mild pain (National Health and Medical Research Council 2005).
Box 12.1 shows the physiotherapy key points with regard to analgesia.
Box 12.1 Physiotherapy key points with regard to analgesia
 Always check vital signs, particularly respiratory rate and blood pressure, as hypotension is a common side effect of pain management. Most important in position changes.
Always check vital signs, particularly respiratory rate and blood pressure, as hypotension is a common side effect of pain management. Most important in position changes. If a patient has had a spinal block or epidural alwaysassess motor and sensory function of the lower limbs, especially before upright mobilization.
If a patient has had a spinal block or epidural alwaysassess motor and sensory function of the lower limbs, especially before upright mobilization. Ask the patient if they need to use their PCA or PCEA before a physiotherapy treatment session or ask about a bolus dose of analgesia before treatment.
Ask the patient if they need to use their PCA or PCEA before a physiotherapy treatment session or ask about a bolus dose of analgesia before treatment. Always liaise with medical and nursing staff before treating the patient and know the local guidelines for mobilizing patients with an epidural in situ.
Always liaise with medical and nursing staff before treating the patient and know the local guidelines for mobilizing patients with an epidural in situ.PCA, patient-controlled analgesia; PCEA, patient-controlled epidural analgesia
Effects of the surgical process on respiratory function
The intra- and postoperative periods are frequently associated with alterations in pulmonary function (Craig 1981). Furthermore, altered physiological function of the respiratory system is an expected finding, especially after upper abdominal and thoracic surgery (Ford et al 1993). The combined effects of the GA, postoperative pain, recumbency, immobility and administration of drugs after surgery lead to several respiratory abnormalities.
Lung volumes
The characteristic abnormality of respiratory mechanics following major surgery is a restrictive ventilatory defect manifest by changes in vital capacity (VC) and functional residual capacity (FRC) (Wahba 1991). The VC can reduce to 40% of preoperative values, while the FRC may gradually reduce to be 70% of preoperative value at 24 hours postoperatively. These changes may persist for 5–10 days following surgery (Craig 1981). The timing of greatest reduction in FRC, while varying between studies, is generally on the first or second postoperative day. In morbidly and even mildly obese patients there is a significant reduction in FRC compared with patients within the ideal weight range (Jenkins & Moxham 1991). Although most other lung volumes also reduce following major surgery, it is thought that the reductions in FRC represent the most clinically important changes because of the functional consequences. The alteration in lung volumes after lower abdominal and laparoscopic surgery is less pronounced.
Functional residual capacity and closing capacity
FRC may be affected by a number of factors. It is linearly related to height and is 10% less in females for the same body height (Nunn 1993). The FRC is affected by gravity and therefore body position. In supine the abdominal contents push the diaphragm cephalad, reducing intrathoracic volume and FRC. In normal subjects FRC is reduced by approximately 500–1000 ml upon adopting the supine position (Macnaughton 1994). It is highest in standing and reduces with recumbency (Fig. 12.3). A change in body position from bed to sitting in a chair increased the FRC by 17% in 10 patients following upper abdominal surgery.
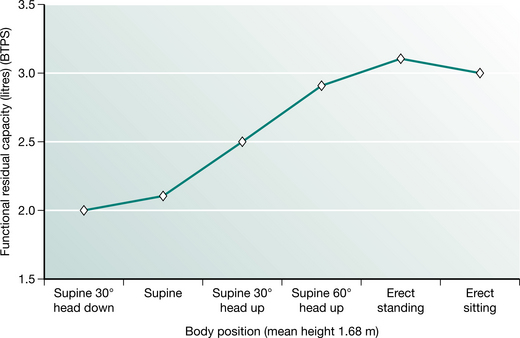
Figure 12.3 Functional residual capacity in different body positions.
(Adapted from Nunn 1993 p 55 with permission from Butterworth-Heinemann, Oxford)
The relationship of FRC with the closing capacity of the lungs explains the functional significance of perioperative reductions in FRC. Closing capacity (CC) is defined as the lung volume at which dependent airways begin to close, or cease to ventilate (Macnaughton 1994). The small airways (less than 1.0 mm diameter) in the periphery of the lung are not supported by cartilage and are therefore influenced by transmitted pleural pressures. Normally the transpulmonary pressure or distending pressure is less than atmospheric, producing a positive pressure, which distends the lungs. Breathing at lower lung volumes produces a higher pressure in gravity-dependent lung regions. This produces a negative distending pressure and causes small, unsupported airways to narrow or close, resulting in reduced ventilation. The relationship between FRC and CC is an important determinant of dependent airway closure. If the CC exceeds the FRC, as it does during tidal breathing in the upright position in people over 70 years of age, then dependent lung regions underventilate, resulting in  /
/ mismatch and hypoxaemia. The relationship between CC and FRC is responsible for the reduction in arterial oxygen tension (PaO2) with increasing age. Closing capacity increases with age due to loss of elastic lung tissue. It also increases in chronic lung disease and with cigarette smoking, due also to changes in lung elasticity. In combination with these increases in CC, any factor which at the same time reduces FRC will significantly affect the relationship between the two volumes, such that dependent airway closure occurs during normal tidal breathing (Macnaughton 1994) (Fig. 12.4).
mismatch and hypoxaemia. The relationship between CC and FRC is responsible for the reduction in arterial oxygen tension (PaO2) with increasing age. Closing capacity increases with age due to loss of elastic lung tissue. It also increases in chronic lung disease and with cigarette smoking, due also to changes in lung elasticity. In combination with these increases in CC, any factor which at the same time reduces FRC will significantly affect the relationship between the two volumes, such that dependent airway closure occurs during normal tidal breathing (Macnaughton 1994) (Fig. 12.4).
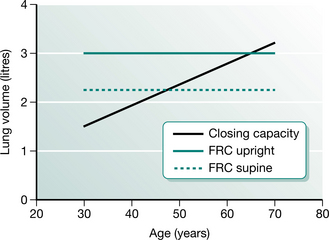
Figure 12.4 The relationship between functional residual capacity (FRC) and closing capacity.
(Adapted from Nunn 1993 p 56 with permission from Butterworth-Heinemann, Oxford)
Anaesthesia, surgery and recumbency reduce FRC. The reduction in FRC together with possible increases in CC in some subjects may account for the regional changes in ventilation that occur perioperatively and lead to reduced compliance, altered ventilation and perfusion ( /
/ ), arterial hypoxaemia and atelectasis from absorption of trapped gas behind closed airways. Postoperative hypoxaemia is inevitable, but usually subclinical, and like FRC, is usually lowest on the first and second days after surgery. Therefore supplemental oxygen is routinely given postoperatively (Craig 1981, Fairshter & Williams 1987).
), arterial hypoxaemia and atelectasis from absorption of trapped gas behind closed airways. Postoperative hypoxaemia is inevitable, but usually subclinical, and like FRC, is usually lowest on the first and second days after surgery. Therefore supplemental oxygen is routinely given postoperatively (Craig 1981, Fairshter & Williams 1987).
The mechanisms for the perioperative reductions in FRC and VC in upper abdominal surgery (UAS) are thought to be: mechanical disruption of the thorax and abdomen, absence of spontaneous sighs, shallow breathing, pain and inhibition of diaphragmatic function (Wahba 1991). Abdominal distension, presence of a nasogastric tube and the use of analgesics to control postoperative pain may impact on ventilatory function.
Mucociliary clearance
Mucociliary clearance is a major function of the airway epithelium. This important function depends both on the physicochemical properties of the airway mucus and on the activity of the cilia (Kim 1997). Anaesthesia, intubation, mechanical ventilation reduced lung volumes and reduced cough effectiveness perioperatively present a significant insult to the mucociliary escalator. A summary of the perioperative contributions to altered mucociliary clearance is given in Box 12.2.
Respiratory muscle function
Diaphragmatic excursion has been shown in research literature to be reduced following abdominal and thoracic surgery and postoperative pain may contribute to diaphragm dysfunction. This is associated with reduced VC, changed pattern of ventilation to predominately rib cage movement rather than abdominal movement and postoperative hypoxaemia. The reduced function may last up to 1 week following surgery. Reflex inhibition of the phrenic nerve may occur after UAS, but research findings are inconsistent. The breathing pattern observed after abdominal surgery may act as a protective mechanism by splinting the abdomen, allowing faster healing of abdominal incisions and reducing the risk of peritoneal infection (Ford et al 1993).
Postoperative pulmonary complications
One of the aims of physiotherapy in the perioperative period is to counteract the adverse pulmonary changes produced as a result of the surgical process (Stiller et al 1994). The factors described above; dependent atelectasis, hypoxaemia and altered V , although occurring in most patients, can lead to clinically significant PPC in some patients after surgery. Two basic theories have been proposed to explain the pathogenesis of PPC following major surgery. Firstly, regional hypoventilation and blockage of airways by mucus and, secondly, absorption of alveolar air distal to a mucus plug in the proximal airways, may lead to eventual collapse unless fresh air enters through collateral channels (Marini 1984). Regional hypoventilation results from reductions in FRC and the altered relationship between FRC and CC together with diaphragmatic dysfunction postoperatively as discussed above. The precise sequence and relative contributions of each of the two mechanisms for developing PPC is unclear. It is possible that they vary among patients and that both alveolar hypoventilation and secretion plugging coexist to contribute to postoperative lung changes (Denehy & Browning 2007). Other risk factors that may predispose to increased risk of mucus plugging may be a history of cigarette smoking, weak cough, prolonged intubation, presence of a nasogastric tube and prolonged postoperative atelectasis (Smith & Ellis 2000).
, although occurring in most patients, can lead to clinically significant PPC in some patients after surgery. Two basic theories have been proposed to explain the pathogenesis of PPC following major surgery. Firstly, regional hypoventilation and blockage of airways by mucus and, secondly, absorption of alveolar air distal to a mucus plug in the proximal airways, may lead to eventual collapse unless fresh air enters through collateral channels (Marini 1984). Regional hypoventilation results from reductions in FRC and the altered relationship between FRC and CC together with diaphragmatic dysfunction postoperatively as discussed above. The precise sequence and relative contributions of each of the two mechanisms for developing PPC is unclear. It is possible that they vary among patients and that both alveolar hypoventilation and secretion plugging coexist to contribute to postoperative lung changes (Denehy & Browning 2007). Other risk factors that may predispose to increased risk of mucus plugging may be a history of cigarette smoking, weak cough, prolonged intubation, presence of a nasogastric tube and prolonged postoperative atelectasis (Smith & Ellis 2000).
The definition of PPC can include atelectasis or pneumonia (atelectasis and collapse are terms that are often used interchangeably). Significant PPCs have been defined as complications that alter the patient’s clinical course (O’Donohue 1992). PPC may be defined by using radiological and bacteriological criteria, clinical signs and symptoms, or a combination of these (Pasquina et al 2006). The definition of PPC impacts on the incidence obtained. Using only radiological evidence of atelectasis for example, gives a higher incidence of complications than using a combination definition. To date, no valid definition has been established and as a result the definitions used are variable.
The clinical signs and symptoms of PPC may include the following:
 arterial desaturation (measured using a pulse oximeter), often defined as <90% on two consecutive days
arterial desaturation (measured using a pulse oximeter), often defined as <90% on two consecutive days radiological evidence of atelectasis or pneumonia (routine chest X-rays are not common after surgery)
radiological evidence of atelectasis or pneumonia (routine chest X-rays are not common after surgery) raised oral temperature (febrile is >37.5°C), often defined as >38°C on more than one consecutive day as a raised temperature on the first day after surgery is a common finding resulting from the surgical insult
raised oral temperature (febrile is >37.5°C), often defined as >38°C on more than one consecutive day as a raised temperature on the first day after surgery is a common finding resulting from the surgical insult abnormal lung auscultation (given that the majority of patients have some dependent atelectasis, reduced breath sounds are commonly found in the first 2 days after surgery)
abnormal lung auscultation (given that the majority of patients have some dependent atelectasis, reduced breath sounds are commonly found in the first 2 days after surgery) prescription of an antibiotic specific for lung infection (many patients are given routine antibiotics immediately postoperatively depending on type of surgery).
prescription of an antibiotic specific for lung infection (many patients are given routine antibiotics immediately postoperatively depending on type of surgery).The incidence of postoperative atelectasis is reported to be 70% following UAS but the incidence of clinically significant complications is reported to range from 5% to 20% (Denehy et al 2001). There is a lower reported incidence following cardiac surgery of around 5%–7% (Brasher et al 2003, Pasquina et al 2003). The incidence following thoracic surgery varies but is reported to be 8–32% (Gosselink et al 2000). A higher incidence of 16% (Law & Wong 2006) to 30% (Gosselink et al 2000) is reported in patients following oesophageal surgery.
Risk factors for postoperative pulmonary complications
Advances in operative technique and postoperative patient management have led to surgery of increasing complexity being performed routinely in patient populations with more severe comorbidities. Estimation of surgical risk is therefore important for all health professionals involved in the management of surgical patients. Surgical risk is the probability of morbidity and mortality secondary to the presence of pre-, intra- and postoperative risk factors. This discussion will be limited to the development of PPC that most affects physiotherapists. Assessment of risk of developing PPC is important for the physiotherapist as it allows prioritized respiratory care for high-risk subjects and more appropriate use of often scarce resources in physiotherapy staffing. Surgical complications such as wound breakdown, bleeding, renal failure and other respiratory problems such as development of pulmonary embolus (PE) will not be discussed in detail. However, it is important to consider a PE in differential diagnosis of a respiratory complication such as pneumonia. The symptoms of both may be quite similar and include pleuritic chest pain, moderate to severe hypoxaemia, breathlessness and fever. Anticoagulation with heparin and then warfarin is indicated for PE. The identification of any pain in the calf on assessment is also important as it may indicate a deep vein thrombosis (DVT), although clinical diagnosis of DVT is unreliable with 50% of patients with DVT on venography showing symptoms.
Several patient characteristics are associated with an increased risk of developing complications. There is a large volume of literature published on this topic. In a systematic review (Fisher et al 2002), 40 variables were reported as possible risk factors for patients having non-thoracic surgery. There have been attempts to find a group of risk factors (model) that predict most complications in a particular patient population; several different models currently exist and none provide perfect prediction. The most common patient, operation and postoperative factors considered to increase risks of developing a PPC are described below. In physiotherapy research, a weighted model was developed to predict the risk of patients having abdominal surgery developing PPC (Scholes et al 2006). Five main risk factors (all these risk factors occurring together in one patient) were identified in this model, which predicted 82% of patients who developed a PPC in a population of 268 patients having upper abdominal surgery. Patients predicted as high risk were eight times more likely than those predicted to be at low risk of developing a PPC. The risk factors identified were:
In addition to the risk factor model above, a systematic review of non-cardiopulmonary surgery (Smetana et al 2006) reports good evidence for the following risk factors to increase incidence of PPC:
Procedure-related risk factors that were supported by good evidence were thoracic, abdominal, emergency and prolonged surgery. There is fair evidence for significant intraoperative blood loss, oesophageal surgery and abnormal chest radiograph. However, in this systematic review, which presents the highest level of evidence, there was good evidence that the following were not important risk factors: obesity, asthma, hip and gynaecological surgery (lower abdominal surgery). These results challenge some traditional views, especially that of obesity being considered a risk factor. The results from this systematic review support the risk factors identified in the model by Scholes et al (2006). In patients having oesophageal surgery, age, operation duration and location of tumour in the proximal oesophagus were identified in one study as risk factors for PPC in 421 patients (Law et al 2004).
Box 12.3 shows key points in the surgical process.
Box 12.3 Key points in the surgical process
 Functional residual capacity (FRC) is reduced perioperatively as a result of anaesthesia, surgery and recumbency.
Functional residual capacity (FRC) is reduced perioperatively as a result of anaesthesia, surgery and recumbency. The altered relationship between FRC and closing capacity is an important determinant of dependent airway collapse.
The altered relationship between FRC and closing capacity is an important determinant of dependent airway collapse. Ventilation/perfusion (
Ventilation/perfusion ( /
/ ) mismatch and arterial hypoxaemia commonly occur after major surgery, although increases in CO2 are rare unless patients are narcotized.
) mismatch and arterial hypoxaemia commonly occur after major surgery, although increases in CO2 are rare unless patients are narcotized. Clinically relevant postoperative pulmonary complication (PPC) may develop in a subset of patients having major surgery.
Clinically relevant postoperative pulmonary complication (PPC) may develop in a subset of patients having major surgery.TYPES OF SURGERY
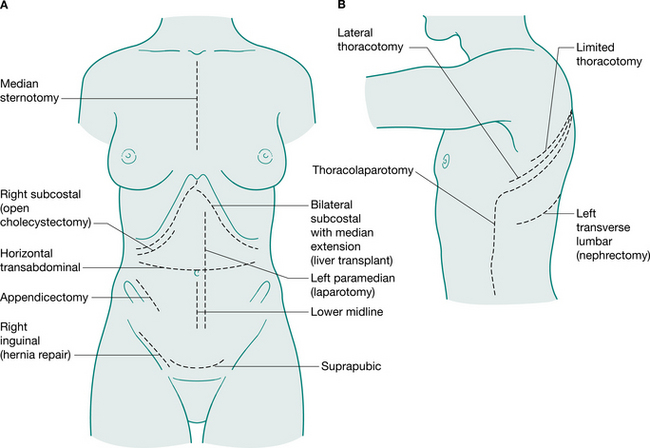
Generally, surgical incisions are placed to optimize access to the target organ (Fig. 12.5). Understanding surgery involves an appreciation of the anatomy of the abdominal and thoracic organs and the muscles and bony structures surrounding them. An appreciation of nomenclature also aids understanding descriptions of operations. The prefix of words can help to locate the surgery: for example, enter- relates to small intestine, gaster- to stomach, pneum- to lung. Surgical procedures may also be named for the person who first performed or reported them: for example, Nissen fundoplication, a wrap of fundus of the stomach around the intra-abdominal oesophagus; a Whipple’s procedure, a pancreaticoduodenectomy; and Hartmann’s procedure, a sigmoid colectomy with a colostomy.
Understanding ‘endings’ of words also helps to work out the type of surgery (Table 12.3).
Table 12.3 Surgical terminology
| Ending | Meaning | Example |
|---|---|---|
| -tomy | Cut, cut out | Thoracotomy |
| -ect | Outside or extra | Gastrectomy (removal of stomach) |
| -stom | Mouth | Colostomy (an opening between the colon and skin) |
| -rraphy | Sew or suture | Herniorraphy (hernia repair) |
| -plasty | Mould or shape | Thoracoplasty (removal of ribs to collapse underlying diseased lung which reshapes the thorax) |
| -plico | To fold | Fundoplication (a wrap or fold of fundus of stomach) |
| -scop | To look at | Mediastinoscopy (look at mediastinum) |
Abdominal surgery
Laparoscopy involves insufflation of the peritoneal cavity with CO2 gas (pneumoperitoneum), insertion of a camera through a 5–10 mm subumbilical incision and inspection of the abdominal contents using the transmitted picture and a monitor. Commonly three ports are used to introduce the instruments and perform the procedure. The technique is performed under general anaesthesia and the most common laparoscopic technique is for the removal of the gall bladder (cholecystectomy), but many other procedures are now performed this way including hernia repair, appendicectomy, splenectomy and oophorectomy (Harris 2006). The term minimal access surgery has been used to reflect the fact that the operations themselves are the same, but the surgical approach is less invasive, which impacts on the postoperative recovery of the patient. It has been well established in the literature that laparoscopic cholecystectomy is associated with a low incidence of PPC (Sharma et al 1996).
Until the publication of a large randomized controlled trial (RCT) in 2004, there was a moratorium placed on laparoscopic cancer surgery owing to concerns regarding the oncological outcomes. This large (1200 cases) RCT reported that that there were no differences found in tumour recurrence using laparoscopic compared with open surgery. The benefits of this minimal access surgery have been reported, in a systematic review, to lead to lower morbidity, reduced postoperative pain, faster recovery of respiratory function, earlier recovery of bowel function and shorter length of hospital stay (laparoscopic patients were discharged a mean of 1.7 days earlier). Laparoscopic surgery, however, took 30% longer to perform (Tjandra & Chan 2006). There is an expanding interest in laparoscopic colorectal surgery but more research on the longer-term outcomes and standardizing surgical expertise are needed. Indeed, the more recent trend toward early postoperative rehabilitation also reduces length of hospital stay with improved patient quality of life after surgery. Therefore the integration of early ‘fast tracking’ rehabilitation with laparoscopic colorectal surgery may be required to fully evaluate the justification of the application of this surgery on a larger scale (Kehlet & Kennedy 2006).
A narrative literature review conducted by Olsen (2000) concluded that routine prophylactic chest physiotherapy is not necessary after laparoscopic upper gastrointestinal surgery such as fundoplication and vertical banded gastroplasty. The efficacy of physiotherapy in other forms of laparoscopic surgery such as colorectal surgery has not been investigated. A survey found that 58% of physiotherapists in Australian hospitals where laparoscopic colorectal surgery is performed routinely assess and treat these patients postoperatively (Browning, personal communication, 2006). However, future research examining the need for physiotherapy in this patient group is recommended.
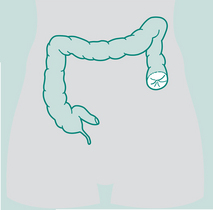
Abdominal surgery includes all operations involving the abdominal viscera. Colorectal and hepatobiliary surgery are most commonly encountered by physiotherapists, since they generally involve an incision above the umbilicus and are considered UAS (Celli et al 1984). Risk of PPC is greater in UAS than in lower abdominal operations such as hysterectomy. Many colorectal procedures are performed to remove cancer, colorectal cancer being the most common cause of cancer death (in non-smokers) in many Western countries. Conditions such as diverticulitis and ulcerative colitis are also common reasons for surgical intervention. Hepatobiliary procedures are performed for both malignant and benign diseases of the biliary tree. These include operations involving the liver, pancreas, spleen, duodenum, bile duct and gall bladder. Box 12.4 describes several commonly encountered abdominal operations.
Box 12.4 Some commonly performed abdominal surgical procedures
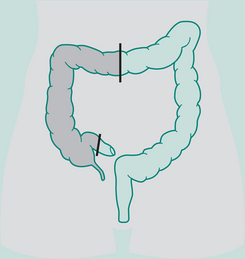
Right hemicolectomy
Indications: Ca right colon, terminal ileum
Incision: Right paramedian, midline, right oblique
Continuation restored by: Anastomosis of ileum to transverse colon
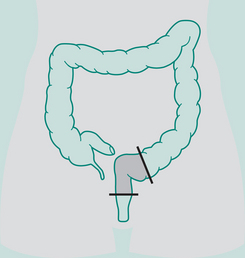
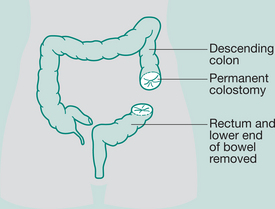
Abdomino-perineal (AP) resection/sigmoid colostomy
Indication: Ca lower portion large bowel and rectum, ulcerative colitis
Incisions: Laparotomy and perineal
Ileostomy
Most are permanent (small bowel)
Indications: Ulcerative colitis, Crohn’s disease, Ca of bowel
Incision: Left paramedian or midline
Operation: Continent pouch ileostomy
Reservoir constructed out of distal ileum: removal of large colon and rectum
Outlet from reservoir is arranged as a valve so that fluid cannot escape on to the abdominal wall
Indications: Ca, trauma, Crohn’s disease, to rest bowel
Incision: Depends on site of colostomy
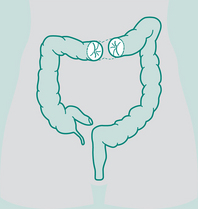
Types of sigmoid colostomy permanent (performed for abdomino-perineal resection – Ca rectum)
Double-barrelled colostomy
Both loop distal and proximal are opened, may be permanent or temporary depending on disease
Loop colostomy
Usually formed in transverse colon. Loop of bowel brought out through incision, plastic
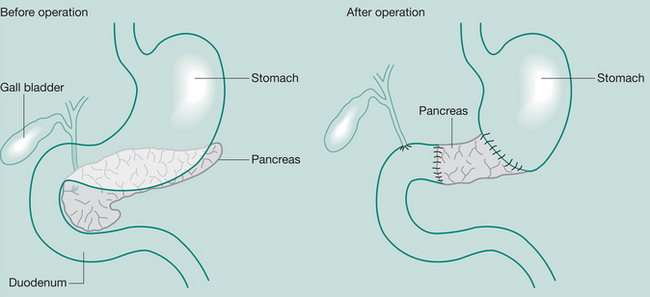
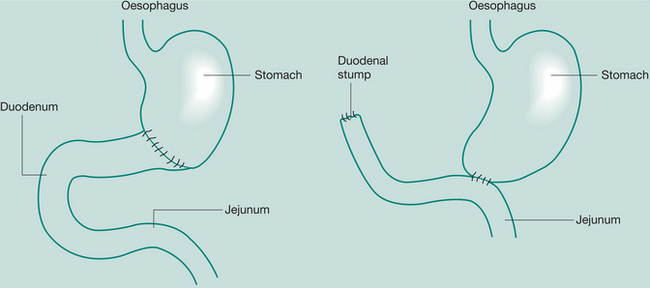
Whipple’s procedure (pancreaticoduodenectomy)
May be required when severe pancreatitis is confined to the head of the gland or in Ca
Gastrectomy
Removal of portions of the stomach
Indications: (partial) peptic ulcer, Ca distal stomach; (total) Ca
Incisions: Upper vertical – midline, paramedian, transverse or oblique.
If thoracic extension for oesophagogastrectomy is involved, a left thoracotomy is performed.
If upper oesophagus is involved, a right thoracotomy is used
Partial gastrectomy, Bill Roth I:
Anastomosis between severed end of duodenum with partially closed end of stomach
Thoracic surgery
Thoracic surgery encompasses topics related to disorders of the chest wall, pleural space, lungs, oesophagus, mediastinum and chest trauma (Smith 2006). It has developed extensively in the past 50 years and now also includes lung transplantation (Chapter 15), video-assisted thoracoscopic surgery (VATS) and lung volume reduction surgery.
Most commonly, removal of part or all of the lung is performed to remove a carcinoma. Of over 3000 lung resections performed in 27 European centres, two-thirds of these were for lung cancer (Berrisford et al 2005). In Australia, the total number of new cases of lung, tracheal and bronchial cancers in 2005 was 9000 and this is predicted to increase (particularly in females) by 30% in 2011 with an ageing population. Lung cancer was the fourth most common cancer in both men and women in Australia in 2003 and presents a significant disease burden (AIHW & AACR 2007). It is reported to be the leading cause of cancer-related death in men and women worldwide (Hassan 2006).
Carcinoma of the lung
Cigarette smoking is the single most common predisposing factor for lung carcinoma, but other factors such as environmental or occupational exposure to hydrocarbons or asbestos are also implicated. There are several different pathological types of lung carcinoma; these are divided into non-small cell and small cell carcinoma. The non-small cell types are squamous cell, adenocarcinoma, large cell and adenosquamous carcinoma. Of these, squamous cell and adenocarcinoma are most common, making up approximately 80% of all lung cancers (Smith 2006). Small cell carcinomas are the most malignant and make up about 10% of presentations of lung cancers. Extrathoracic spread is common at the time of presentation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree