Fig. 3.1
Sudden cardiac death rate according to stage of chronic kidney disease. Rates shown as events per 1,000 patient-years and were as follows: eGFR ≥60 ml/min, 3.8 (95 % CI: 0, 8); eGFR 15–59 ml/min, 7.3 (95 % CI: 2, 13); eGFR <15 not on dialysis, 12.5 (95 % CI: 5, 20); and dialysis, 24.1 (95 % CI: 14, 34) (Reprinted by permission from Macmillan Publishers Ltd: [KIDNEY INTERNATIONAL] (Pun et al. [5]), © (2009))
Atrial and ventricular arrhythmias are also a very common phenomenon in individuals with CKD. The prevalence of atrial fibrillation in hemodialysis patients is increasing (reported rates are 7–20 %), thus increasing the risk of ischemic stroke and death [6]. Multiple small studies have assessed ventricular arrhythmias in hemodialysis patients with reported rates of 5–75 % during HD treatments [7, 8].
The very high rate of cardiovascular death and the substantial contribution of SCD to these rates in patients with CKD and ESRD is not fully explained by the high prevalence of traditional cardiac risk factors including hypertension, smoking, diabetes and dyslipidemia [9, 10]. This chapter focuses on the cardio-renal interplay that predisposes to SCD and examines the evidence for prevention and intervention.
Risk Factors and Mechanisms
Sudden cardiac death occurs in patients who encounter stressors to a vulnerable myocardium (Fig. 3.2) [11]. The predisposition for arrhythmias and SCD increases as CKD deteriorates. Also, the hemodynamic and metabolic milieu present in patients undergoing dialysis is multifaceted, complex and malignant [12]. These patients develop cardiomyopathy with diastolic and systolic dysfunction, fibrosis and vascular disease. In addition, sympathetic overactivity, inflammation, abnormalities in mineral metabolism, electrolyte shifts, hyper/hypovolemia, and QT prolongation together predispose to arrhythmias and SCD [13, 12].
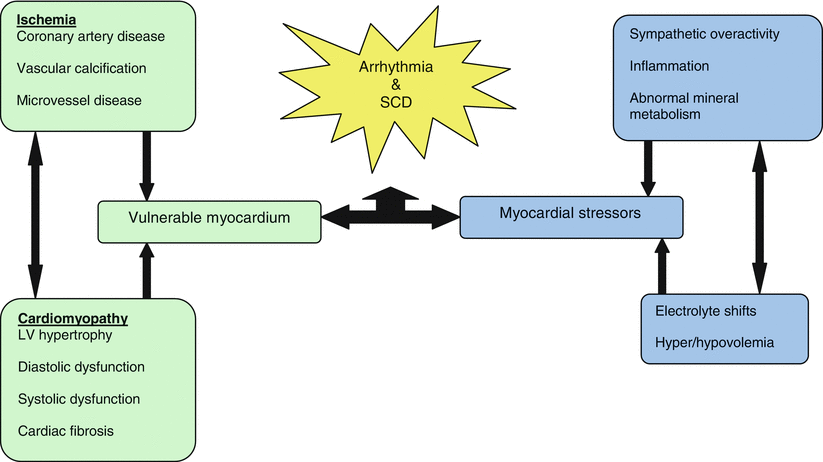

Fig. 3.2
The etiology of sudden cardiac death occurs in uremic kidney disease
Coronary Artery Disease
In the general population, coronary artery disease (CAD) is an important cause of SCD. In patients with CAD, for each 10 ml/min decrement in eGFR the risk of SCD increased by 11 % [5]. CAD is present in at least 38 % of the prevalent dialysis population [14]. In these patients, the intra- and post-dialytic period is associated with a high frequency and lengthy persistence of ventricular arrhythmias [15]. However, unique factors other than CAD likely contribute to the increased risk of SCD in ESDR patients [1].
Arteriosclerosis and Arterial Calcification
Arteriosclerosis occurs frequently in patients with CKD and ESRD and is characterized by diffuse dilatation, wall hypertrophy and stiffening of the aorta and large central arteries. Arterial stiffening leads to increased systolic and decreased diastolic blood pressure. This widened pulse pressure has two major results; (1) left ventricular hypertrophy from increased systolic blood pressure and (2) diminished coronary and subendothelial blood flow during diastole [16, 17]. Arteriosclerosis and increased vascular stiffness in adults with CKD is more related to age, systolic blood pressure, diabetes and vascular calcification rather than uremic toxicity [18].
Arterial calcification is associated with cardiovascular death and SCD in patients with ESRD [19]. In CKD and ESRD patients, metastatic vascular calcification occurs as a result of an elevated calcium phosphate product, diabetes, dyslipidemia, oxidative stress, uremia, hyperphosphatemia and elevated promoters of calcification in the vessel wall [20]. Two major types of arterial calcification occur: patchy calcification of the intima associated with atherosclerotic plaques and diffuse calcification of the media, in the absence of cholesterol deposits, associated with arteriosclerosis [20]. Vascular calcification worsens as CKD progresses [18], resulting in a loss of arterial elasticity, increase in pulse wave velocity, left ventricular hypertrophy, decreased coronary artery perfusion and myocardial ischemia [20].
Microvessel Disease
Compared to non-uremic controls, an autopsy study of a small group of deceased dialysis patients reported inadequate capillary growth relative to the degree of cardiac hypertrophy [21]. Therefore, uremic patients are at increased risk of ischemia related arrhythmias due to the capillary: myocyte mismatch [1]. Furthermore, microvessel disease may occur as a result of diabetes, hypertension or calcium phosphate deposition.
Myocardial stunning during hemodialysis may contribute to myocardial damage because reduction in regional wall motions have been observed, presumably the result of transient hypoperfusion causing ischemia, followed by reperfusion necrosis [22].
Cardiomyopathy and Cardiac Fibrosis
Approximately half of patients with congestive heart failure (CHF) have CKD [23]. Furthermore, the Case Mix Adequacy Study of the USRDS found that 59 % of patients with SCD also had CHF [24]. CHF is the major manifestation of left ventricular dysfunction, and both diastolic and systolic dysfunction occurs frequently in ESRD.
Left ventricular hypertrophy (LVH), both concentric and eccentric [25, 26], is very common in patients with CKD, and in up to 75 % of patients starting HD [27, 28]. Hypertension, arteriosclerosis and aortic stenosis predispose to concentric LVH whereas anemia, blood volume expansion, and the arteriovenous fistula predispose to eccentric LVH [13]. Progressive LVH and left ventricular dilatation occurs after dialysis is initiated [28]. As myocytes are lost systolic dysfunction may occur. An Italian study of a small cohort of dialysis patients revealed that LVH is associated with increased risk of SCD [29]. This is consistent with what is seen in the general population: in the Framingham Heart Study, SCD increased for each 50 g/m increase in left ventricular mass (adjusted for height) [30]. Systolic dysfunction on starting dialysis is an adverse predictor of subsequent CHF and death [31].
The uremic heart is associated with increased interstitial myocardial fibrosis on post mortem examinations. Gadolinium enhanced magnetic resonance imaging also revealed images consistent with fibrosis in patients on dialysis [32]. On a molecular level, activation of growth factors, proto-oncogenes, plasma noradrenalin, cytokines, and angiotensin II also play a role in promoting LVH, fibrosis and apoptosis [12]. These changes cause slowing of conduction across diseased tissue and the development of both atrial and ventricular arrhythmias – likely triggers for SCD [1].
Sympathetic Overactivity
Coronary artery disease, arteriosclerosis, arterial calcification, microvessel disease, cardiomyopathy, and cardiac fibrosis all increase the vulnerability of the myocardium to stressors. In kidney failure the sympathetic nervous system is inappropriately activated which begins in the early stages of CKD and increases with worsening eGFR [33]. Multiple mechanisms for this phenomenon have been proposed. Kidney ischemia associated with progressively damaged renal parenchyma triggers sympathetic nerve activity, a theory supported by the fact that sympathetic overactivity was normalized after the correction of renal artery stenosis in patients [34]. In addition, patients undergoing hemodialysis (N = 18) had higher levels of sympathetic discharge; hemodialysis patients with bilateral nephrectomy had normal rates of sympathetic discharge [35, 36]. Vasoconstriction of the kidney also activates the renin-angiotensin-aldosterone system leading to elevated levels of angiotensin II and as a result more sympathetic activation. Another novel mechanism for sympathetic overactivity involves renalase – a monoamine oxidase. Renalase is released from the kidney and is responsible for catecholamine catabolism. Levels of renalase are reduced in ESRD with a consequent increase in catecholamine levels [37].
Sympathetic overactivity results in enhanced sympathetic and norepinephrine release, predisposing to hypertension, LVH, heart failure and arrhythmias [38].
Inflammation
Patients on dialysis are “at high risk for chronic inflammation due to the non-physiologic nature of the dialysis procedure, infections, vascular access, and multiple co-morbid conditions” [39]. The highest tertiles of the inflammatory markers C-reactive protein (CRP) and interleukin-6 (IL-6) were associated with a doubled risk of SCD compared with the lowest tertiles in a cohort study of 1,041 dialysis patients. The same study reported a decrease in serum albumin (a manifestation of inflammation) was associated with a 1.35 fold increased risk of SCD [39]. In patients with CKD, elevated levels of inflammatory mediators induce the production of reactive oxygen species that may accelerate vascular atherosclerosis [40]. Inflammation could trigger sudden cardiac death by multiple mechanisms including: (1) direct effect on the myocardium and the electrical conduction system (2) cytokine induced plaque instability and (3) aggravation of sympathetic tone [39].
Abnormalities in Mineral Metabolism
Hyperphosphatemia is a common disturbance of mineral metabolism in patients with ESRD affecting up to 40 % of patients undergoing hemodialysis [41]. SCD has been associated with hyperphosphatemia in a large study of 12,833 patients [42]. After adjusting for several known mortality predictors, higher levels of serum phosphate (PO4 >6.5 mg/dl) had a 41 % greater risk of death resulting from CAD and a 20 % greater risk of death resulting from sudden death compared to patients with serum PO4 between 2.4 mg/dl and 6.5 mg/dl.
It is unclear how hyperphosphatemia increases the risk of SCD. It is postulated that hyperphosphatemia affects intracellular handling of calcium and therefore triggers electrical instability [43]. In addition it provokes secondary hyperparathyroidism, smooth muscle proliferation, vascular calcification, and coronary atherosclerosis [44]. Hyperparathyroidism is a predictor of death in ESRD [45]. The calcimimetic, cinacalcet, reduces PTH levels and in elderly patients (≥65 years old) significantly lowered the risk of death or a major cardiovascular event, suggesting that in this sub-group of patients hyperparathyroidism is an important risk factor [46].
Electrolyte Shifts and Hypervolemia
During the hemodialysis session, there is a rapid change of the extracellular potassium concentration, leading to secondary shifts between the intracellular and extracellular compartments, and cellular membrane instability [47]. In a study from the United Kingdom, 40 patients had continuous EKG holter monitoring during the hemodialysis session, using a dialysate potassium concentration of 1 mmol/L. Lower post-dialysis potassium concentrations were associated with more premature ventricular complexes (PVCs) and complex ventricular arrhythmias [48]. Another study showed that dialysate potassium concentrations of 0 or 1 mmol/l were a significant risk factor for cardiac arrest [47]. A case control study involving 502 cases of cardiac arrest compared with matched controls reported a dialysate potassium of <2 meq/L was associated with a two-fold increase in sudden cardiac arrest (Fig. 3.3) [49]. In the same study, low calcium dialysate (<2.5 meq/l) was associated with cardiac arrest (OR 1.88, CI 1.28–2.76).
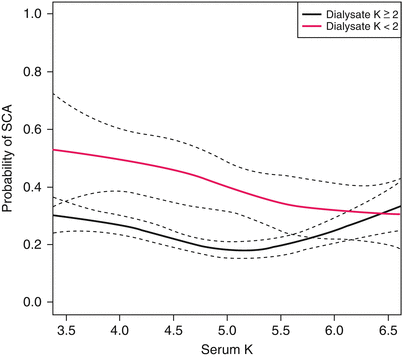

Fig. 3.3
Probability of sudden cardiac arrest (SCA) by dialysate potassium concentration (2 meq/l, black line; <2 meq/l, red line) and last recorded serum potassium. Dashed lines represent 95 % confidence intervals. Difference in risk is greatest for lower levels of serum potassium, and the risk difference decreases as serum potassium increases. No significant advantage of low potassium dialysate is observed at any level of serum potassium (Reprinted by permission from Macmillan Publishers Ltd: [KIDNEY INTERNATIONAL] (Pun et al. [49]), © (2011))
Ultrafiltration of consistently large volumes during hemodialysis was a risk factor for peridialytic cardiac arrest [49]. Significantly increased rates of dysrhythmia and cardiac arrest occur following the long interdialytic period, typically on Monday, particularly in those on dialysis three times per week [49, 50]. The increased rates of arrhythmia and cardiac death following 3 days without dialysis may be due to hyperkalemia, increased blood volume or changes in divalent ion levels [43, 13].
QT Prolongation
QT prolongation (a sign of abnormal electrical signal propagation) increases the risk of fatal arrhythmia, notably in the form of torsades de pointes – a polymorphic ventricular tachycardia [51]. Patients with advanced CKD and ESRD have prolonged QTc (corrected QT) presumably resulting from left ventricular hypertrophy and uremic cardiomyopathy [52]. Furthermore, during the hemodialysis session itself, in 68 nondiabetic patients without EKG evidence of LVH, the QTc interval increased [53].
Dialysate containing lower concentration of potassium and calcium was associated with the prolongation of the QT interval [54]. Also, higher pre-dialysis calcium and lower serum calcium levels after dialysis were associated with greater increases in QTc intervals [54]. These data suggest that abnormalities of calcium associated with derangement of the QT interval may predispose to SCD.
Evidence for Best Management
The above case presentation is a familiar one in the nephrology world, but sometimes SCD comes as a shock to care providers. The patient had no symptoms of IHD or CHF. So why did this man die suddenly? Postmortem analysis revealed that he had a myocardium susceptible to ventricular fibrillation including coronary artery disease, uremic cardiomyopathy and fibrosis. Risk factors for SCD included hyperphosphatemia, hyperparathyroidism and systemic inflammation with elevated CRP and hypoalbuminemia. Also, his death came on a Monday following his longest interdialytic period. Moreover, he was being dialyzed with a 1 mmol/L potassium bath, had a relatively high ultrafiltration volume and a short dialysis time.
Could this SCD have been prevented? Unfortunately, the risk factors for sudden cardiac death in dialysis patients are not fully understood and prevention studies are limited.
Beta-Adrenergic Blockers
B-blockers may prevent SCD in dialysis patients by improving blood pressure, decreasing arrhythmias, decreasing ischemia, and diminishing sympathetic overactivity [36], but little randomized trial data using clinical outcomes in large numbers of patients is available. Observational studies are limited by the difficulty in controlling for selection bias.
A Japanese cohort involving 2,075 dialysis patients examined beta-blocker use and mortality [55]. There was a lower rate of all cause mortality in the B-blocker group (p < 0.007). However, the study was limited due to the small number of patients in the treatment group (247) and the total duration of B-blocker use was difficult to determine. Also, it must be noted that the Japanese dialysis population is very unique as it has the lowest dialysis mortality rate in the world, patients receive more total dialysis and are very compliant with their prescribed medical care [56]. A post hoc analysis of the HEMO study assessed beta-blockers for prevention of SCD in 1,747 hemodialysis patients in which 39 % had ischemic heart disease and 12 % had moderately severe CHF [57]. Beta-blocker use was not associated with a decrease in SCD. However, a benefit with B-blockers was seen in those with pre-existing ischemic heart disease (p = 0.03). This study was limited by its observational nature but a propensity-matched comparison strengthened the statistical analysis. The only prospective randomized control trial to assess beta-blocker use and mortality in dialysis patients examined carvedilol in 103 dialysis patients with cardiomyopathy [58]. At 2 years, there was a significant difference in mortality with 30 deaths (51.7 %) in the carvedilol group compared with 41 (73.2 %) in the placebo group (HR 0.51, 95 % CI 0.32–0.82, p < 0.01). There were also fewer all-cardiovascular deaths (29.3 vs. 97.9 %, HR 0.32, 95 % CI 0.18–0.57, p < 0.00001). Furthermore the treatment group had fewer deaths from fatal MI and stroke.
With regards to mortality benefit after cardiac arrest, a large retrospective study of 43,200 dialysis patients reported B-blockers increased the odds of survival by 40 % [59]. Another study examined he safety profile of B-blockers in dialysis patients and reported no significant increase in the risk of bradycardia or hyperkalemia [60].
In conclusion, as there is limited randomized trial data pertaining to the use of B-blockers in patients with CKD, the indications for these agents derived from patients without renal disease should probably be applied to those with CKD.
Renin-Angiotensin System (RAS) Blockade
Blockade of the renin-angiotensin system could reduce the incidence of SCD in dialysis patients by curbing sympathetic overactivity and by limiting cardiac remodeling. In one small, randomized control trial of 80 dialysis patients with no evidence of cardiac disease, the use of the angiotensin receptor blocker candesartan was associated with a reduced incidence of cardiac events and mortality versus placebo [61]. Conversely, a prospective controlled trial with the angiotensin converting enzyme inhibitor fosinopril in ESRD patients had no significant impact on mortality. However, after adjustment for risk factors, there was a trend towards decreased cardiovascular events [62]. With regards to mortality benefit after cardiac arrest, the large retrospective study on 43,200 dialysis patients mentioned above reported that the use of ARB and ACE inhibitors was also associated with better survival after cardiac arrest [59]. Again, due to the lack of good evidence on the blockage of the renin-angiotensin system, the indications for these agents derived from patients without renal disease or from trials to slow kidney disease progression should be applied to those with CKD. However, caution must be taken because of the risk hyperkalemia. Also, the overuse of RAS blockers in ESRD patients may detrimentally reduce residual urine output. Certainly, caution is necessary in the use of combined RAS blockade with ACE inhibitor/ARB, in view of the harms reported in the recent VA NEPHRON-D trial [63].
Dialysis Dose and Avoiding low Potassium Dialysate
Although a higher dialysis dose improves the uremic milieu particularly through reducing phosphate levels and deceasing levels of inflammation and middle molecules, there was no major clinical benefit with high dialysis dose or high flux membrane use [64]. However, longer dialysis time may provide hemodynamic benefits, with less fluctuation in blood pressure and better ultrafiltration. More frequent dialysis sessions than the conventional three times per week was associated with significant reduction in death or LVH [65].
As detailed above, rapid shifts of potassium and the use of low potassium dialysate are associated with arrhythmia and SCD. Therefore, the dialysis prescription should be evaluated on a regular basis [1]. Our patient should probably have been prescribed a 2 meq/L potassium bath and longer dialysis to manage the increases in interdialytic weight gain.
Management of Mineral Abnormalities
Poor clinical outcome data from randomized control trials exists to inform clinicians on treatment of hyperphosphatemia with calcium and non-calcium based phosphate binders. Meta-analysis suggests a survival benefit for non-calcium based phosphate binders compared to calcium-based binders but the evidence is weak [66]. Use of vitamin D derivatives is driven by biochemical consideration because little clinical outcome data has been reported. Evidence from the randomized trial of cinacalcet versus placebo in hemodialysis patients with moderate to severe hyperparathyroidism reveals a clinical benefit in the prevention of cardiovascular events in patients ≥65 or in the prevention of severe unremitting hyperparathyroidism in all patients [46, 67].
Implantable Cardiac Defibrillator
There are no randomized trials that assess the use of ICD implantation for primary or secondary prevention in CKD patients. Much of the present data highlights persistently high mortality rates in this patient population despite ICD use. A longitudinal, prospective study followed 146 patients with chronic heart failure, an ICD, mean ejection fraction <29 % and CKD in 75 % of patients. CKD was independently associated with increased mortality [68]. A retrospective cohort of 9,528 dialysis patients with ICDs reported a very high mortality rate after ICD implantation at (45 deaths/100 patient years). This is double the adjusted mortality rate of unselected dialysis patients in 2008. Thirteen deaths/100 patient years were due to arrhythmia. Furthermore, there was a very high complication rate with infections and bacteremia occurring at rates in excess of 1 infection/2 person years of follow-up [69]. A meta analysis of 2,516 patients with 89 receiving dialysis observed an increase in mortality in ICD patients receiving dialysis compared to those who were not (RR 2.67, 95 % CI 1.68–4.25, p < 0.0001), implying limitation in the effectiveness of ICDs in reducing overall mortality in this population. Mechanisms of death other than SCD are important in the dialysis population as only 4/27 patients with documented death died from arrhythmic deaths. In this study, a proposed mechanism for an arrhythmic death despite ICD implantation was inappropriately high defibrillation thresholds observed in dialysis patients (i.e. ventricular fibrillation that could not be terminated by high ICD energy levels) [70].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


