Epidemiology
SCD accounts for ˜350,000 deaths annually in the United States, which represents an incidence of about 0.1% to 0.2% per year in the adult population or 1 to 2 deaths per 1,000 person-years.
1 The incidence of sudden death in adolescents and young adults is far lower, about 0.003% per year, about 25% of which are unexplained, even with autopsy (sudden arrhythmic death syndrome [SADS]).
2 SCD can also occur in young, otherwise healthy-appearing athletes, where the incidence ranges from 1:3,000 person-years to 1:9,17,000 person-years, with the highest incidence seen in African American male basketball players.
3 Men, in general, have been reported to have at least a two- to fourfold increased risk when compared with women, depending somewhat on the population being considered.
Causes and mechanisms of SCD, discussed in detail below, differ by age group (
Tables 141.1,
141.2,
141.3,
141.4). In individuals more than 40 years of age, most causes are acquired, with coronary atherosclerosis and its
consequences responsible for the death in more than 7 of every 10 cases (
Table 141.3). Hypertensive heart disease is the second most common cause and has appreciable overlap with atherosclerotic heart disease, given the common risk factors.
In those <40 years of age, congenital disease is the most common cause of SCD, including anomalous coronary artery origin and genetic cardiomyopathies (
Tables 141.3 and
141.4).
5 Myocarditis is also much more common among the young.
Approach to the Heart in SCD
It is especially important to rule out noncardiac natural and unnatural causes of death before determining the manner and cause of sudden deaths. In the case of hospital-based or private autopsies, the manner is determined to be natural prior to autopsy and documented by the death certificate. Any case of suspicious or undetermined manner of death is referred to a medical examiner or coroner’s office for clearance.
The standard approach to the heart is appropriate for the vast majority of cases of SCD. Given that most arrhythmogenic substrates for sudden death are within the myocardium (not the specialized conduction system), the heart should be cut serially along the short axis to maximize the area visualized and allow for accurate and complete description of the identified lesion(s).
The ventricular cavities should be measured (excluding trabeculations and papillary muscles). Additionally, the ventricular wall thicknesses including those of the free walls and septum (again, excluding trabeculations and papillary muscles) should be measured. Because recent ischemic foci (<12 hours) are usually not evident grossly, a careful evaluation of the coronary vessels, including their course, is necessary, which often necessitates removal of the coronary arteries from the heart and subsequent decalcification. Tetrazolium salts may be useful in evaluating for early ischemic changes (see
Chapter 14). The coronary ostia should be carefully inspected for anomalous origins and patency.
Because many causes of SCD are underlying chronic conditions, there is no way to prove causation in an individual case, other than by exclusion. Exceptions to this rule are cardiac tamponade caused by aortic rupture or myocardial infarct, or saddle pulmonary embolism (technically not sudden cardiac death), which are never incidental findings. Acute occlusive coronary thrombosis and spontaneous coronary artery dissection are virtually never incidental, because of their acute nature. Acute myocardial infarctions are also seldom incidental, although uncommonly seen, because death generally occurs before histologic manifestations become manifest.
Routine evaluation of the cardiac conduction system study has fallen out of favor because arrhythmias in the face of negative autopsy
are generally caused by channelopathies that have no gross or histopathologic correlate. Nevertheless, evaluation of the cardiac conduction system can occasionally yield relevant findings. In cases with an antemortem history of heart block, abnormalities of the region of the atrioventricular node (AVN) (including involvement by sarcoidosis) can be seen. Additionally, in cases of myxomatous mitral valve disease, abnormalities of the atrioventricular conduction system have been described.
6,7
Dissection of the Sinoatrial and Atrioventricular Nodes
The sinoatrial node is located on the lateral aspect of the junction of the superior vena cava and the trabecular portion of the right atrium, in the superior aspect of the sulcus terminalis (which corresponds internally to the crista terminalis). Dissection involves identification of junction between the muscular and venous portion of the right atrium, with cross sections to identify the sinoatrial nodal artery around which the nodal tissue resides. Histologically, the node consists of small specialized cardiomyocytes within fibrous and elastic tissue.
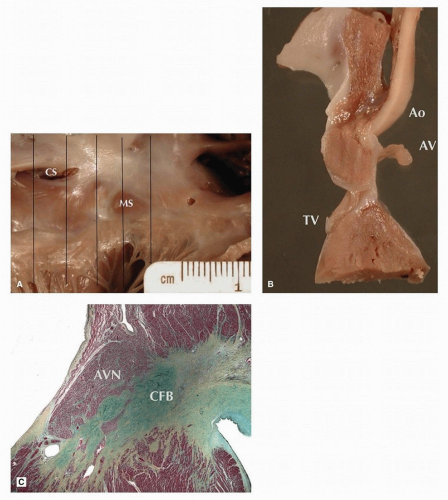
The AVN is located within the triangle of Koch immediately posterior to the central fibrous body (CFB) (
Fig. 141.1). The dissection is generally approached from the right atrium, identifying the coronary sinus posteriorly, annulus of the tricuspid valve anteriorly, and membranous septum superiorly. The posterosuperior limit is formed by the tendon of Todaro, a microscopic bundle of fibrous tissue that is not seen grossly extending from the junction of the valves of the inferior vena cava and coronary sinus toward the commissure between the septal and anterior tricuspid valve leaflets. Sections are taken from inferior
to superior, with consecutive sampling of the compact AVN, penetrating bundle of His, and proximal left bundle branch (LBB) and right bundle branch (RBB).
Histologically, the nodal tissues should be identified, with attention to inflammation, tumor, and increased collagen. Other abnormal findings include dysplastic changes of the atrioventricular nodal artery, which have been associated with sudden death, and infiltrative processes such as amyloidosis. Rarely, necrosis of myocardium in the crest of the ventricular septum can be seen in association with isolated small vessel disease at the base of the heart, as well as small vessel disease associated with mitral valve prolapse.
7,8 In patients with heart block, either acquired or congenital, there may be scarring in the area of the node and the branching bundle.
Postmortem Genetic Testing
In selected autopsy cases of SCD where no pathologic substrate could be identified, including unexplained drowning deaths, sequencing candidate genes may be of some utility.
9 Testing in these individuals is important because it can serve as proband identification in families and allow for efficient screening of at-risk individuals. Such testing should be performed in a clinical laboratory. In the case of underlying channelopathies, identification of a causal mutation will be the only way of recognizing the disease. In a series of SCD cases in which an underlying channelopathy was identified, fewer than one-third of patients had a history of cardiac events.
9Postmortem genetic testing in those with cardiomyopathy has only uncovered a causal mutation in ˜10% of cases. This relatively low number may be, in part, explained by wide diversity of mutations that can cause these diseases (often kindred specific) and our somewhat rudimentary variant classification techniques. Patients dying with hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy, and arrhythmogenic cardiomyopathy showed mutations in
MYBPC3,
MYH7,
LMNA,
PKP2, or
TMEM43 genes, distributed across all three groups.
10
Specific Causes of SCD
The cause of SCD is typically attributed to one or more
morphologic substrates that increase the risk of electrical instability that can lead to a fatal dysrhythmia. Because of uncertainty in the relationship between the cause of death and the individual substrate in many cases, causes of SCD have been classified as certain, highly probable, and uncertain, with the majority falling in the highly probable group.
11Chronic occlusions of the epicardial coronary arteries impart an increased risk for sudden death, likely due to the increased potential for transient ischemic events during periods of increased myocardial oxygen demand. When secondary myocardial changes, such as remote infarctions (replacement-type fibrosis) and/or hypertrophy, are present, the designation of ischemic heart disease can be applied.
By convention, at least 75% cross-sectional narrowing (grade 4 stenosis) of an epicardial coronary artery needs to be found in order for the coronary lesion to be considered a likely cause of death. In cases of anomalous origin, especially of the left main coronary artery, the explanation for sudden death is also not clear in most cases, as the lesion is chronic and present from birth. There is often a history of exertion during or prior to death resulting in increased oxygen demand and histologic change of prior ischemic episodes. Aside from the origin of the coronary arteries, the ultimate course is important as well.
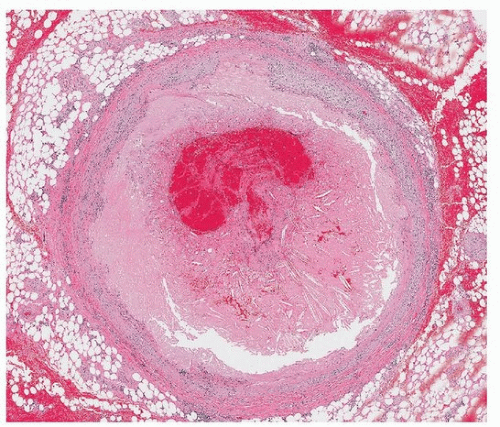
Coronary thrombosis, resulting in sudden occlusion of an epicardial artery, can arise in the setting of atherosclerosis (
Fig. 141.2). This can cause focal ischemia or infarction and an ensuing arrhythmia. Because ischemic myocardium cannot be identified at autopsy until histologic changes are manifest (at least several hours), the ultimate cause (myocardial ischemia) will often not be identified but rather inferred by identification of the said thrombus. Uncommon causes of sudden narrowing of an epicardial artery include spontaneous coronary artery dissection and embolic phenomenon, the latter of which can derive from thrombus or tumor.
Heart failure is known to increase the risk for ventricular arrhythmias and may serve as an intermediary mechanism behind SCD. Importantly, however, it is not technically a “cause” of death. Rather, it represents a constellation of symptoms resulting from the inability of the heart muscle to effectively pump blood to meet the metabolic demands of the body. This results from an underlying pathology, such as ischemic heart disease, hypertensive heart disease, valvular heart disease, or a cardiomyopathic state. In most cases, there is a history of heart disease, and the patient is taking cardiac medications.
Myocardial inflammation can be the only finding to explain SCD. The most common pattern is diffuse lymphocytic myocarditis with associated myocyte necrosis, usually seen in younger patients and caused by viruses and autoimmune disease. Predominantly, interstitial infiltrates with relatively little necrosis are characteristic of hypersensitivity (or drug-related) myocarditis, consisting of a mix of histiocytes, eosinophils, and occasional neutrophils. The role of focal myocarditis as a cause of sudden death is debated, and often ascribed as a contributing or possible cause.
Myocardial scarring is frequent in SCD and usually seen as a consequence of cardiomyopathy, coronary artery disease, myocarditis, or sarcoidosis. The pattern and size of scars are important to note, as well as the pattern of replacement or interstitial fibrosis. Small amounts of subendocardial fibrosis are common in dilated or hypertrophied hearts, and interstitial collagen is a normal finding in some areas of the myocardium, including near the CFB and near insertions of the atrioventricular valves.
Cardiomegaly, typically diagnosed when heart weight is more than 50% above the expected mean, can result from many of the abovementioned conditions (e.g., hypertension, chronic valvular disease, cardiomyopathy, and ischemic heart disease). The presence of cardiomegaly is associated with an increased risk of SCD.
Most (up to 80%) children dying suddenly (of apparent SCD) do not have an anatomic substrate identified by traditional gross and microscopic evaluation.
12 The proportion of so-called autopsy-negative SCD (SADS) is ˜30% when young adults are considered
2,12 and is about 12% for all adults.
11 Cases of autopsy-negative SCD are likely to decrease with broader availability of advanced genomic and proteomic ancillary studies.