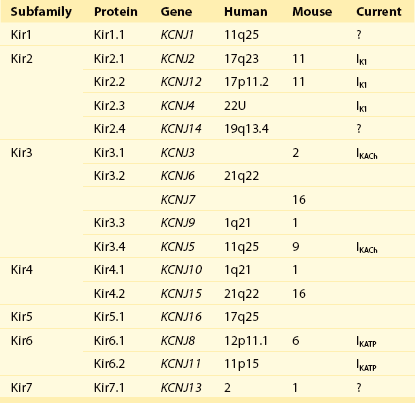
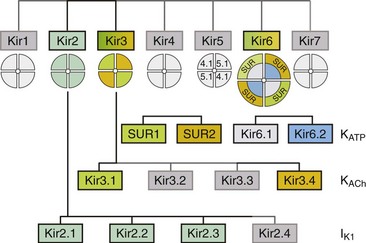
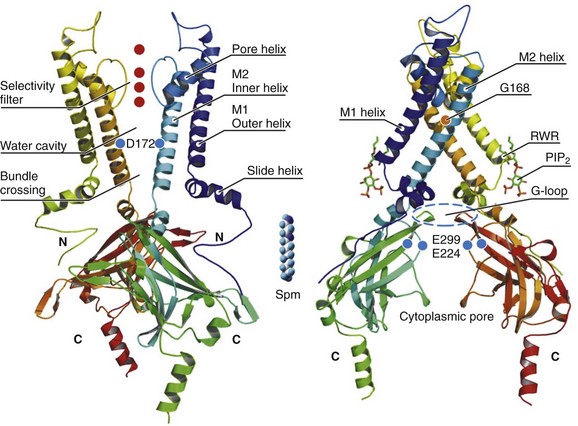
4 Inwardly rectifying potassium (Kir) channels are important for stabilizing the resting membrane potential, establishing the threshold of excitation, and modulating the repolarization phase of the cardiac action potential.1 Inward rectification is a process in which the conductance of the Kir channel increases with membrane hyperpolarization, but decreases with depolarization to potentials positive to the potassium equilibrium potential. In essence, Kir channels behave as “bio-diodes,” preferentially passing current in one direction. The molecular basis of rectification in Kir channels is a physical occlusion of the ion permeation pathway by depolarization-induced movement of intracellular cations, such as magnesium and polyamines.1 Only few Kir channels, however, display strong rectification.2 Strong rectification enables the Kir channels to stabilize the resting membrane potential, as well as to protect the cell from an excessive loss of K+ ions during the plateau phase of an action potential.1 The molecular correlates of these channels are primary subunits encoded by the KCNJ family of genes.3 Mutations in KCNJ genes have been associated with various channelopathies,2 demonstrating the importance of Kir channels in normal cardiac excitation. This chapter will focus on three well-studied Kir channels in the myocardial cells: the classical inward rectifier potassium channels (IK1), the acetylcholine-activated potassium channels (KACh), and the adenosine triphosphate (ATP)-sensitive potassium channels (KATP). Additional information on the topics covered in this chapter can be obtained from recent review articles.2,4 Channels belonging to the Kir family are structurally and functionally different from voltage-gated potassium channels.2,4 The genes that encode Kir channels are ascribed the KCNJ nomenclature and are categorized into seven subfamilies based on the gene products (Kir1-7; Table 4-1; Figure 4-1). In general, Kir channels consist of homomeric or heteromeric complexes of the respective Kir subunits, but as will be discussed, functionality of the resulting channels depends partially or solely on interactions with additional (accessory) proteins.2 From a functional perspective, there are three distinct classes of cardiac Kir channels (IK1, IKACh, and IKATP). As will be discussed later in this chapter, based on the degree of inward rectification the underlying channels can be considered as strong (IK1; KACh) or weak (KATP) inward rectifiers.2 Table 4-1 Diversity of α-Subunit Proteins in the Family of Inward Rectifier Potassium Channels (Modified from Nerbonne JM, Nichols CG, Schwarz TL, et al: Genetic manipulation of cardiac K+ channel function in mice: what have we learned, and where do we go from here? Circ Res 89:944–956, 2001). Figure 4-1 The family of inward rectifier potassium channels. All members of the Kir family share significant structural similarity, but only Kir2 and Kir3 subfamilies represent channels carrying classical strongly rectifying currents. Four members of each Kir2 and Kir3 subfamilies were cloned in mammals. Heteromeric assemblies of Kir2.1, Kir2.2, and Kir2.3 subunits underlie IK1 current, and heteromeric assembly of Kir3.1 and Kir3.4 subunits underlies IKACh current. Weakly rectifying KATP channels are composed of pore-forming Kir6.1 and Kir6.2 subunits and auxiliary SUR1and SUR2 subunits. (Modified from Anumonwo JM, Lopatin AN: Cardiac strong inward rectifier potassium channels. J Mol Cell Cardiol 48:45–54, 2010.) The Kir2 subfamily consists of five members (Kir2.1-Kir2.5), of which only Kir2.1-Kir2.4 are expressed in the mammalian heart. Kir2.5 is expressed in the fish. As shown in Table 4-1, the following genes encode the mammalian cardiac Kir channels: KCNJ2, KCNJ12, KCNJ4, and KCNJ14, for Kir2.1 through Kir2.4, respectively. There is evidence that in the mammalian heart Kir2.1-Kir2.3 isoforms are expressed in cardiac myocytes, and that Kir2.4 is probably only expressed in neuronal cells. It is well established that members of the Kir2 subfamily underlie IK1, although the subunit composition varies among species and cell types, and channel complexes are likely formed as hetero-tetrameric structures. In recent years, x-ray crystallographic structures of both bacterial and mammalian homologs of several Kir channels have been obtained.5 Figure 4-2 highlights some important and common features of Kir channel structure based on the results of work with Kir2.1 and Kir2.2 mammalian channels.6 It is now firmly established that Kir channels are tetramers of distinct subunits, each having two transmembrane domains (M1 and M2), relatively small N-terminal, and large C-terminal cytoplasmic domains, and a pore-forming structure between M1 and M2 (see Figure 4-2). The pore structure contains pore helix directed toward the conduction pathway and the characteristic GYG (or GFG) motif, also known as K+ channel signature sequence, that contributes to the selectivity filter in all potassium channels. The M1 and M2 transmembrane domains in each subunit are arranged as an antiparallel coiled-coil and make contact with each other. Kir2 channels have a negatively charged amino acid (D172 in Kir2.1) located in approximately the middle of the pore, which has a critical role in the phenomenon of inward rectification discussed later. Figure 4-2 Architecture of a typical Kir channel. X-ray structure of chicken Kir2.2 crystallized in the presence of PIP2 (Accession code 3SPI, available in the Protein Data Bank; from Hansen et al.6). Only two opposing subunits are shown for clarity. Right, The structure is rotated approximately 90 degrees to better present the intracellular ion conduction pathway and bound molecules of PIP2. Numbering of the amino acids corresponds to that in Kir2.1. Red circles represent K+ ions in the selectivity filter. Blue circles show the location of three residues most critical for inward rectification. Spermine molecule (Spm) is shown in the middle at the same scale as the Kir2.2 structure. Analysis and visual presentation of the structures were performed using DeepView Swiss-PdbViewer and PyMol software. The so-called slide helix formed by the N-terminus region just preceding the M1 helix is another unique and important regulatory feature of both bacterial and mammalian Kir channels.7 This helix intercalates between the inner leaflet of the plasma membrane and cytosol likely because of its amphiphilic nature. The important functional role of the slide helix is highlighted by the fact that many loss-of-function mutations associated with Andersen-Tawil syndrome (LQT7) are located in the slide helix. A large C-terminal domain provided by four Kir2.x subunits consists primarily of β-strands and potentially strongly interacts with a smaller N-terminal domain. As shown in Figure 4-2, C- terminal domain forms a large intracellular vestibule, approximately 30 Å in length, for easy ion passage and likely provides binding sites for various intracellular agents. The cytoplasmic domain harbors a number of residues (e.g., E224 and E299 in Figure 4-2) known to contribute to inward rectification. A membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) is an important structural and regulatory component of Kir channels.8 In the membrane, PIP2 likely interacts with hydrophobic amino acids on both M1 and M2 transmembrane helices as well as with a well-conserved (among Kir channels) RWR motif located just at the end of the M1 domain (see Figure 4-2). The interaction of PIP2 with a specific region in the CTD leads to an approximately 6 Å translation of the entire CTD toward the membrane associated with the movement of the M1 helix, which ultimately leads to the opening of the gate.6 The key experiments conducted in mid-1990s with the first cloned members of the Kir2 subfamily have clearly demonstrated that almost all essential properties of classical strong inward rectification could be explained by a voltage-dependent block of the channel by ubiquitous intracellular organic cations, the polyamines.9 Micromolar concentrations of free polyamines (primarily spermine and spermidine) are sufficient to reproduce the degree of rectification observed in native cells. The strength of rectification varies among the different members of the Kir subfamily, and although every Kir channel shows some degree of inward rectification, they can be broadly grouped as either strongly rectifying (Kir2 and Kir3) or weakly rectifying (Kir1 and Kir6) channels. It is well established that the time-dependent activation of strong, inward rectifiers during membrane hyperpolarization reflects the exit of polyamines (primarily sperimine) from the pore. Early work with cloned Kir channels established that strong inward rectification by intracellular polyamines depends on three negatively charged residues located in the second transmembrane domain (D172; the rectification controller; see Figure 4-2) and in the C-terminal tail (E224 and E299; Kir2.1 amino acid [aa] numbering; see Figure 4-2). Positively charged polyamines enter the Kir channel pore to physically occlude it, a process aided by negative charges provided by aspartate and glutamate residues. Both electrophysiologic and structural data are consistent with the idea that channel block occurs in two sequential steps. A weakly voltage-dependent (shallow) blocking step involves entry of polyamine into the Kir2.1 pore at a site provided by a ring of negative charges (E224 and E299) in C-terminus. A more strongly voltage-dependent blocking step reflects the movement of polyamine to its deep binding site near D172 residue. It is also believed that the strong voltage dependence of the polyamine block arises not only from the high valency (z) of polyamines (z≈4 for spermine) but also from a displacement (push) of K+ ions through the pore.10 Among the polyamines, spermine is the most voltage-dependent blocker and also has the highest potency for blocking the channel. A characteristic feature of Kir2.1 and Kir3 channels is a flexible cytoplasmic pore-facing G-loop (see Figure 4-2) that forms a girdle around the central axis of the Kir channel.11 It was estimated that this girdle constricts the ion permeation pathway to approximately 3 Å. Mutations in the G-loop were shown to disrupt inward rectification. In addition to the previously described E224 and E299 residues, it was shown that A255 and A259 located farther away from the pore axis are also involved in channel rectification. Electrophysiologic experiments using cysteine modifications in the pore region of a mutant Kir6.2 channel (N160D; equivalent to D172 in Kir2.1) showed that spermine binds at a deep site beyond the rectification controller residue D172, a site that is close to the extracellular mouth of the pore. In another study, refinement of the crystal structures of bacterial KirBac1.1 and KirBac3.1 allowed identification of the shallow polyamine binding sites at the cytoplasmic interface between the two subunits.12 These observations notwithstanding, the precise mechanism involved in rectification is still being worked out, and the exact location of polyamine binding sites in Kir channels is still a controversial issue. Consistent with their overall significant sequence homology, all Kir2 channels have the three mentioned residues (D172, E224, and E299) important for rectification at the equivalent positions. Recent studies, however, unexpectedly showed that rectification properties are rather different in the three Kir2 isoforms.13 Specifically, the data show that Kir2.2 channels display significantly stronger voltage dependence of rectification than that observed in Kir2.1 and Kir2.3 channels. The voltage dependence of steady-state rectification is different between Kir2.x channels, as are single-channel conductance and kinetics properties of the currents. In particular, polyamine unblock (activation) at negative membrane potentials in Kir2.3 channels is several-fold slower than in Kir2.1 and Kir2.2 channels. Single-channel conductance is smallest in Kir2.3 (approximately 10 pS), medium in Kir.2.1 (approximately 25 pS) and largest in Kir2.2 (approximately 35 pS). Molecular biologic studies are also consistent with location-dependent expression of specific Kir2 isoforms. In particular, real-time reverse-transcriptase polymerase chain reaction analysis of Kir2 transcripts in the human heart showed the following relative expression levels: in Purkinje fibers, Kir2.1 > Kir2.3 > Kir2.2; and in the right ventricle, Kir2.1 > Kir2.2 > Kir2.3; and the sequence was reversed in right atrium, Kir2.3 > Kir2.2 > Kir2.1.14 Information on expression patterns of Kir2 subunits can also be gleaned from functional data using the unique properties of corresponding channels. For example, in cardiac atrial and ventricular myocytes, unitary conductance values display a wide spectrum ranging from 10 to 15 pS (as in Kir2.3 channels) to as high as 40 to 45 pS (as in Kir2.2 channels). Evidence shows that IK1 channels are located not only in the non–T-tubular component of sarcolemma of ventricular myocytes, but also in the intercalated discs and in the T-tubules. For example, accumulation and depletion of K+ only in T-tubules lead to changes in whole-cell IK1.15 IK1 accumulation/depletion phenomena are not observed in atrial cells, which essentially lack T-tubules and in ventricular myocytes, in which T-tubules are removed by osmotic shock.15 Kir2.x and IK1 channels can be regulated in a number of ways.2 Most studies on adrenergic stimulation show that inward IK1 currents are suppressed by activation of both α and β receptors, although opposite effects were also described. In addition, adrenergic regulation is clearly dependent on the type of receptors and subunit composition of the channel. Both isoproterenol and forskolin inhibit IK1 in human ventricular myocytes, suggesting involvement of protein kinase A (PKA)-mediated phosphorylation of underlying Kir2.x subunits. Molecular details of the phenomenon, however, are contradictory. For example, it has been shown that the application of a catalytic subunit of cyclic adenosine monophosphate (cAMP) dependent PKA leads to activation of Kir2.1 channels expressed in Xenopus oocytes, but to Kir2.1 inhibition when the channels they are expressed in a mammalian cell line (COS-7). The data on PKA regulation of native IK1 channels is limited and somewhat controversial; however, most of the studies show that IK1 channels are inhibited by exposure of the cytosolic side of the membrane to purified catalytic subunit of PKA. Studies in exogenous-expressing systems also showed the involvement of PKC in negative regulation of Kir2.1 channels. Consistent with the latter, experiments using human atrial myocytes show that α1-adrenergic stimulation likely reduces IK1 via a PKC-dependent mechanism. Kir channels are also targets for phosphorylation by tyrosine kinases. In Kir2.1 channels, the site of downregulation was targeted to a single Y242 residue in the C-terminus.16 PIP2 is an important component in membrane-delimited second messenger signaling system and a powerful activator of Kir channels.17
Structural and Molecular Bases of Cardiac Inward Rectifier Potassium Channel Function
Background
A Family of Genes Encode Inward Rectifier Potassium Channels
Classical Cardiac Inward Rectifier Potassium Channels
Structure and Function
Kir2 Subfamily Underlies Cardiac IK1
Crystal Structure of Kir2 Channels
Mechanism of Polyamine-Induced Rectification
Rectification Properties Are Related to Electrostatic Interactions in the Cytoplasmic Pore of a Kir Channel
Differential Properties of Kir2.x Subfamily
Cellular and Membrane Localization
Pharmacology and Regulation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Structural and Molecular Bases of Cardiac Inward Rectifier Potassium Channel Function
