Stroke and Transient Ischemic Attack
Abdul Abdullah
Lee H. Schwamm
Clinical Diagnosis of Stroke and TIA
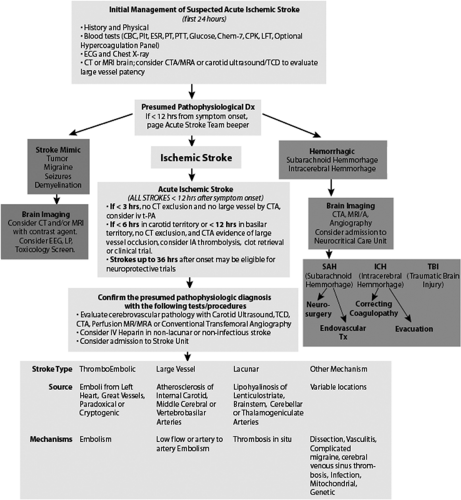
Stroke is a major cause of morbidity and mortality in the United States. It is the third leading cause of death and continues to be a primary cause of long-term disability in the United States. About 700,000 Americans have a new or recurrent stroke each year: 88% of all strokes are ischemic, 9% are due to intracerebral hemorrhage, and 3% are due to subarachnoid hemorrhage (1). Because treatment is available for acute ischemic stroke (AIS), it is critical to have methods for rapidly identifying the stroke, subtype and initiating treatment protocols. An example of such an algorithm can be seen in Figure 9-1 and is also available in a hyperlinked version on our stroke management internet resource at http://www.acutestroke.com.
Although it is important to distinguish ischemic stroke from transient ischemic attack (TIA) because they reflect different clinical syndromes, it is also important to recognize that they frequently reflect the same underlying pathophysiology, namely atherosclerosis or thromboembolism. TIA, sometimes called a “ministroke” in common parlance, is defined as “a sudden, focal neurological deficit that lasts for less than 24 hours (and) is confined to an area of the brain or eye perfused by a specific artery,” whereas ischemic stroke lasts more than 24 hours. Although both of these are clinical syndromes and do not require evidence of brain infarction by neuroimaging, many physicians would classify a transient syndrome with evidence of infarction as an ischemic stroke rather than a TIA. This has led to some attempts at reclassification and to emphasize the importance of treating ischemic stroke and TIA in a similar manner.
Neuroimaging techniques, including CT (computed tomography), MRI (magnetic resonance imaging), and PET (positron emission tomography), have revolutionized our concepts of temporal events that occur in relation to brain ischemia (2). This technology allows neurologists to detect ischemic episodes more accurately and quickly. Efficient discovery of brain infarction is valuable in initiating secondary prevention strategies that will reduce the risk of subsequent debilitating events.
In a study of patients diagnosed with TIA in an emergency department (ED), 10.5% of the patients suffered an ischemic stroke within 90 days of the diagnosed TIA, and of those, 50% had an ischemic stroke within a 24- to 48-hour time period. Increased risk was associated with advanced age, diabetes mellitus, hypertension, and a history of vascular disease (3). Thus, clinicians have proposed a new definition of TIA that takes into consideration the fact that a TIA can result in permanent damage to the brain. The definition is targeted at facilitating brain imaging and rapid intervention (2).
In light of these findings, it is apparent that the urgent evaluation of an ischemic stroke or TIA should not be significantly divergent because both have the potential for substantial short-term deleterious consequences.
Current Recommended Treatments
Thrombolysis in Acute Ischemic Stroke
IV tPA (Intravenous recombinant tissue plasminogen activator-rtPA)
The efficacy of tPA has been evaluated by two major studies: the NINDS tPA trial and the ECASS study (4,5). The NINDS tPA trial was a randomized, double-blind, placebo-controlled trial that demonstrated that patients treated within 3 hours of symptom onset for ischemic stroke had a better clinical outcome at 3 months. This benefit was measured as a 12% absolute and 30% relative greater chance of having minimal or no disability at 3 months in all four outcome measures (NIH Stroke Scale, Barthel Index, Modified Rankin Scale, Glasgow Outcome Scale) as compared with placebo-treated patients. Secondary analysis of the NINDS trial showed statistically significant improvement in the median National Institute of Heath Stroke Scale (NIHSS) score at 24 hours in the tPA group (8 versus 12; P <0.02). All the stroke subtypes benefited consistently from tPA treatment. Rates of symptomatic intracerebral hemorrhage within 36 hours after the onset of stroke were 6.4% in patients who received tPA versus 0.6% for patients who received placebo (P <0.001) (4).
The ECASS trials (ECASS 1, ECASS 2) attempted to increase the time window of treatment with the administration of tPA within 6 hours of symptom onset. The ECASS trials did not meet their primary efficacy endpoints, but meta-analyses confirm the benefit of tPA in the first 3 hours after stroke onset and suggest that tPA may be beneficial between 3 and 5 hours after stroke onset in a subset of patients with preserved brain parenchyma on imaging (5,6).
The major consensus organizations and evidence-based guidelines recommend administering tPA for carefully selected AIS patients within the 3-hour window of established symptom onset time (7,8). In addition, pooled data analysis of three large trials (ATLANTIS [Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke], ECASS, and NINDS) using an adjusted multiple regression model demonstrated that earlier treatment is strongly linked to a larger benefit, and patients should be treated as rapidly as possible (9).
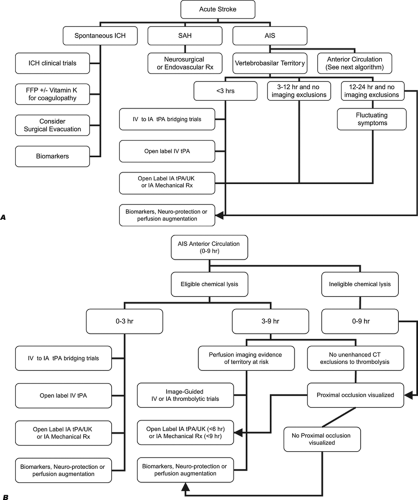
The development of a critical care pathway for thrombolysis in acute stroke treatment attempts to ensure the efficient and equitable delivery of tPA to appropriate patients. Critical care protocols aid in rapid identification and treatment of ischemic strokes, thereby increasing the rates of IV tPA use and decreasing the practice deviations (10,11,12). Our Massachusetts General Hospital (MGH) acute stroke critical pathway for thrombolytic therapy is shown in Figure 9-2. This algorithm is placed
in the context of catheter-based and nonreperfusion strategies in our acute stroke algorithms, as shown in Figure 9-3.
in the context of catheter-based and nonreperfusion strategies in our acute stroke algorithms, as shown in Figure 9-3.
IA Thrombolysis for AIS
PROACT II (Prolyse in Acute Cerebral Thromboembolism II) was the first randomized multicenter trial to show the clinical efficacy of IA thrombolysis in patients with acute stroke of less than 6 hours’ duration caused by middle cerebral artery (MCA) occlusion. The study demonstrated a 15% absolute benefit with 40% of the r-proUK treated group (P = 0.04) achieving independence (Modified Rankin score of 2 or less) at 90 days, compared to only 25% of the control patients. The recanalization rate at the end of treatment was 66% for the r-proUK group and 18% for the control group (P <0.001) (13).
Clinical trials assessing the use of combined IV and IA-tPA are underway. One such pilot study demonstrated that combined IV/IA treatment was feasible and provided better results with respect to recanalization, even though it was not coupled with improved clinical outcomes (14). The latest guidelines suggest that even though IA thrombolysis alone or in combination with IV tPA (also called bridging therapy) is promising, the applicability of this intervention still needs to be validated by randomized studies and should only be performed at centers with dedicated personnel and experienced operators (8).
MERCI for AIS
Previously the only Food and Drug Administration (FDA)-approved treatment for AISs was IV rtPA. However, the FDA recently approved a mechanical embolectomy device (MERCI [Mechanical Embolus Removal in Cerebral Ischemia]) for clot removal within 8 hours of symptom onset. It is a treatment option for patients who do not qualify for r-tPA administration. The primary study outcome for the unblinded, single-arm MERCI trial was the rate of recanalization of the basilar artery, terminal internal carotid artery, or the main middle cerebral artery branches versus control subjects from the PROACT 2 trial (46% versus 18% in an intention to treat analysis). At 90 days, patients with recanalization were more likely to be alive and independent (Modified Rankin score of ≤2) than those without recanalization (15).
Antithrombotics for AIS
Aspirin is the only antiplatelet agent that has been thoroughly evaluated for AIS (7). A placebo-controlled trial of aspirin (325 mg/dL) reported no major decline in rate of neurological deterioration. Patients were enrolled within 48 hours of onset of stroke symptoms and treated for 5 days (16). Administration
of abciximab, an intravenous chimeric mouse–human monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor, has shown promising results when given within 6 hours of stroke onset and is under study (8).
of abciximab, an intravenous chimeric mouse–human monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor, has shown promising results when given within 6 hours of stroke onset and is under study (8).
The meta-analysis of eight trials provides more detailed data regarding the efficacy of antiplatelet agents (17). It essentially shows that, for every 1,000 acute strokes treated with aspirin, there are approximately 7 fewer early recurrent strokes and 13 fewer deaths and/or dependencies (7). Based on these data, current recommendations endorse the use of aspirin within 48 hours of onset of stroke symptoms because the benefits, though small, are significant, particularly in light of safety and cost considerations. However, aspirin and other antithrombotics should not be used within 24 hours of the use of thrombolytic therapies (7,8).
Emergent Carotid Endarterectomy (CEA) for AIS/TIA
The efficacy of emergent CEA for acute ischemic strokes and TIA (18,19) remains unproven, though some studies have shown promising results. One retrospective study of 21 patients for CEA performed within 24 hours of diagnostic workup for TIA or AIS showed the survival rates at 1 and 5 years to be 90% and 62%, respectively (20). In another study of 67 patients (58% strokes, 42% TIA), flow through the internal carotid artery was re-established in 93% of patients (21). Diffusion or perfusion mismatch criteria for the selection of candidates for CEA may lead to improved patient selection (22).
Evidence-Based Interventions in Hemorrhagic Stroke
Surgery or Endovascular Therapy for Aneurysmal SAH (Followed by Nimodipine for Vasospasm)
Incidence of subarachnoid hemorrhage (SAH) from an intracranial aneurysm rupture occurs at a frequency of 6 or 8 per 100,000 person-years (23). The International Subarachnoid Aneurysm Trial (ISAT) compared the safety and efficacy of endovascular treatment of ruptured intracranial aneurysms and conventional neurosurgical treatment (craniotomy and clipping of the ruptured intracranial aneurysm). A total number of 2,143 patients with ruptured intracranial aneurysms were enrolled and randomly assigned to neurosurgical clipping (n = 1,070) or endovascular treatment by detachable platinum coils (n = 1,073). At 1 year, endovascular coiling reduced the relative risk of death and dependence by 22.6% with an absolute risk reduction of 6.9% (24).
SAH may result in cerebral vasospasm, which is the delayed narrowing of large-volume arteries at the base of the brain. Cerebral vasospasm is often coupled with radiographic or cerebral blood flow evidence of reduced perfusion in the distal territory of the affected artery. A number of prospective, randomized trials for the oral agent nimodipine demonstrate that it consistently reduces poor outcome due to vasospasm in all grades of patients (25).
Correcting Coagulopathy in Intracerebral Hemorrhage
Spontaneous intracerebral hemorrhage (SICH) is the most fatal form of stroke with a mortality rate between 30% and 55%, increasing to as high as 67% in patients receiving oral anticoagulant therapy (OAT). There are currently no standardized guidelines for reversal of the anticoagulant effect in the patients with OAT-ICH. Administering 10 mg vitamin K with every treatment to support the supply of prothrombin-dependent clotting factors has been recommended. Other treatment options include fresh frozen plasma (FFP), prothrombin complex concentrates (PCC), and recombinant activated factor VIIa (rFVIIa) (26). Rapid correction of the abnormal International Normalized Ratio (INR) is associated with successful reversal at 24 hours; compared to patients who were not successfully reversed at 24 hours, patients whose INR was successfully reversed within 24 hours had a shorter median time from diagnosis to first dose of FFP (90 minutes versus 210 minutes; P = 0.02). In a multivariate model, shorter time to vitamin K as well as FFP predicted INR correction (27). An example of a clinical protocol for reversal of anticoagulation is presented in the following extract.
MGH Strategy for Correcting Coagulopathy in Intracerebral Hemorrhage
Warfarin
If the patient is on warfarin and the INR is elevated or if the prothrombin time (PT) is elevated in the absence of warfarin therapy physicians at MGH administer vitamin K 10 mg IV over 10 minutes followed by FFP 10 mL/kg over 90 minutes. Vitamin K and FFP must be dosed at once and the team must designate a single physician to take personal responsibility for ensuring that these therapies are administered as fast as possible. Vitamin K should be administered within 5 minutes of the order.
As soon as FFP is ordered, a “runner” should be dispatched to the blood bank to collect FFP, which should be administered as soon as possible.
Standard (Unfractionated) Heparin
The preferred immediate therapy at MGH to correct coagulopathy is to administer Protamine 10 to 50 mg IVP over 1 to 3 minutes.
Low-Molecular-Weight Heparin
Protamine sulfate reverses only about 60% of the antifactor Xa activity of low-molecular-weight heparin and has negligible effects on danaparoid (a mixture of anticoagulant glycosaminoglycans used to treat heparin-induced thrombocytopenia) and fondaparinux (a synthetic antithrombin-binding pentasaccharide with exclusive antifactor Xa activity). Therefore more research is needed to develop a more effective treatment.
Direct Thrombin Inhibitors (Argatroban, Lepirudin, Bivalirudin, Ximelagatran)
There is no specific antidote for these drugs at this time. At MGH, antifibrinolytic agents such as Amicar (EACA) are used at the attending physician’s discretion.
Platelet Disorders
In the cases of thrombocytopenia (platelet count <100,000/μL), platelets are transfused until the platelet count exceeds 100,000/μL. For Von Willebrand syndromes, 0.3 μg/kg DDAVP is administered intravenously over a period of 30 minutes. Phone consult is initiated with a staff member of hematology or transfusion medicine for dosing of VWF factor concentrate.
DDAVP is also of benefit in patients with:
Uremic platelet dysfunction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree