Fig. 8.1
Stephen Hales, D.D., F.R.S. (1677–1761)
Stephen Hales is best known in physiology for his work on blood pressure, which is described in his book Haemastaticks [7]. A modern facsimile reprint is available [9]. This includes an extensive analysis of flows and pressures in keeping with the mechanical Newtonian flavor of his work. Most of his material on respiratory physiology is in his other great book Vegetable Staticks [6]. A modern reprint is available [8]. This, as its name implies, is chiefly about botanical research including an explanation for the rise of sap in plants. The title of this book presumably partly explains why Hales’s contributions to respiratory physiology are so little known.
Hales was a great experimentalist, with an inexhaustible curiosity and drive. This is shown in the first page of Haemastaticks in which he plunges into an experimental account of how he first measured blood pressure in the horse, “In December, I caused a Mare to be tied down alive on her Back, she was fourteen Hands high, and about fourteen Years of Age, had a Fistula on her Withers, was neither very lean, nor yet lusty: Having laid open the left crural Artery about three Inches from her Belly, I inserted into it a brass Pipe whose Bore was one sixth of an Inch in Diameter.” His questioning thrusting inquisitive attitude was typical of the time as evidenced by the stimulating accounts of the meetings of the Royal Society [2]. Hales was elected a Fellow in 1718, and his Vegetable Staticks, published in 1727, had the imprimatur of Isaac Newton who was the President of the Royal Society at that time.
Hales worked in a number of areas of respiratory physiology. First, he was interested in the nature of the respiratory gases, although there was bound to be some confusion in this area until Black, Scheele, Priestley, Cavendish and Lavoisier had identified oxygen, carbon dioxide and nitrogen later in the century. Hales was fascinated by the fact that gases could exist in what he called the elastic and fixed states, i.e., in the gaseous form and as nongaseous compounds. In one experiment he rebreathed air from a bladder for a minute at the end of which he had to stop because of a feeling of suffocation (Fig. 8.2). He showed that during this time about one-thirteenth of the total volume of air was transformed from the elastic to the fixed state, i.e., had been absorbed by the body [6] (experiment CVIII).
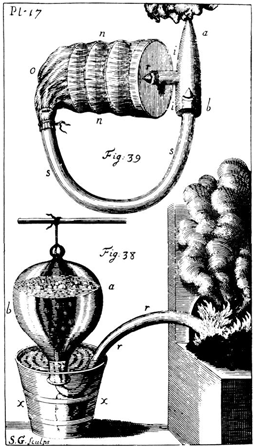

Fig. 8.2
Upper part shows closed circuit for rebreathing with 2 one-way valves (r, b). n is an elastic bladder containing hoops on which were placed pieces of moist cloth to absorb gases. Lower part shows pneumatic trough invented by Hales for collecting gases
He was able to show that the period of rebreathing could be extended if pieces of moist cloth soaked in vinegar or sal tartar (probably made from the sediment of wine casks) were included in the breathing circuit. These presumably absorbed some of the carbon dioxide and this observation prompted Joseph Black to carry out his experiments on caustic lime which led to the discovery (or actually the rediscovery) of carbon dioxide. Hales argued that the increase in weight of these absorbers reflected the amount of gas changed from the elastic to the fixed state. The breathing circuit that he used for these experiments included two one-way valves and would look familiar today (Fig. 8.2). Hales went on to suggest that a similar closed-circuit breathing device could be employed as a respirator by someone who had to enter a noxious atmosphere for a short time.
In connection with these experiments Hales invented the pneumatic trough for collecting gases, and this was to be used extensively by later chemists including Priestley and Lavoisier. This trough can be considered a derivative of the upturned jar which was employed so effectively by the Oxford physiologists, e.g., Mayow, in their experiments on respiration and combustion.
Hales’s observations on the absorption of gases by the lung led him to studies of the morphometry of the lung and its implications for function [6] (experiment CIX). He measured the size of the alveoli in the calf, obtaining a value of one hundredth of an inch or 250 µm. The actual value is about 100 µm [11], so Hales’s measurement using the crude equipment of that time was impressively accurate. He calculated the surface area of the interior of the lungs to be 289 ft2 (about 30 m2) and pointed out that this was 10 times the surface area of a man’s body. He recognized the importance of this large surface area for gas exchange and remarked that the “partitions” in the lung are very thin to allow a “continued succession of fresh air” to be absorbed by the blood. In other experiments he measured the amount of gas in blood by heating the latter in a closed container connected to a gas reservoir and reported that “a cubick inch of Hog’s blood… produced thirty three cubick inches of Air.”
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue



