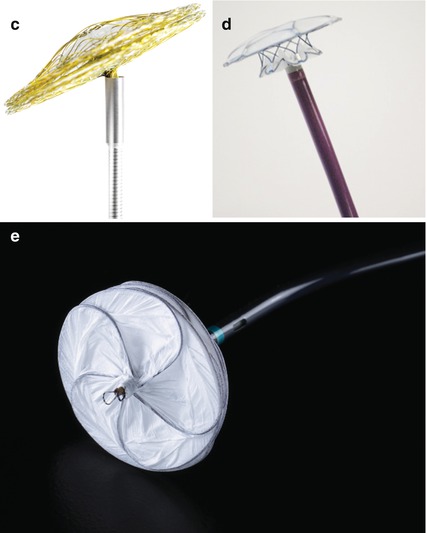
Fig. 26.1
(a) Amplatzer septal occluder (ASO). The red arrow points to the self centring core of the device. (b) Occlutech Figulla occluder, left atrial aspect. (c) Occlutech Figulla occluder, articulation with the delivery cable. (d) Cardia Ultrasept occluder. Showing the left atrial disc and the central portion N.B. the right atrial disc is undeployed within the delivery sheath. (e) Gore septal occluder: left atrial aspect. Note the five interlocking wires forming the frame covered in ePTFE
There are broadly two types of occluder: self-centering (with a core) and the non-self-centering devices (with a thin central stalk). Self-centering devices are most commonly deployed due to their ability to deal with defects of most sizes.
26.2 Anatomic Description
Deficiencies occur in a number of positions within the atrial septum and an understanding of the anatomy is important when considering closure and the potential effects of a device within the heart (Fig. 26.2a). The atrial septum is not symmetrical. When viewed from the right atrial side, a significant proportion of the atrial septum is made up of infoldings of the atrial wall, usually in association with other structures (Fig. 26.2b). From the right, postero-superiorly the septum consists of an infolding of the atrial wall between the superior caval vein and the insertion of the right pulmonary vein into the left atrium (as the heart sits within the chest). Anterior to this the rim of the atrial septum continues behind the aortic root. Inferiorly, the rim of the atrial septum is in contact with the inferior caval vein and anterior to this margin is the interface with the coronary sinus, which in turn is separated from the mouth of the inferior caval vein by the Eustachian ridge and valve (sinus septum). Deeper within the Eustachian ridge runs the tendon of Todaro, one of the three walls of the triangle of Koch, an area of the atrial septum containing several important structures including electrically active tissue (Fig. 26.2c).
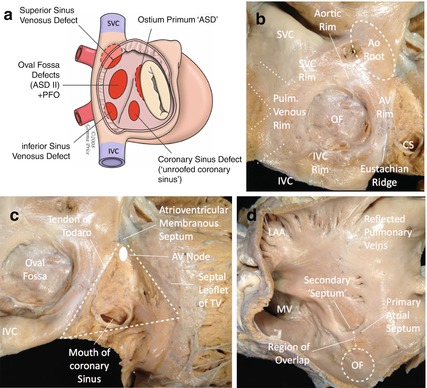

Fig. 26.2
(a) Schematic diagram of defects of the atrial septum, viewed through the right atrium (Image courtesy of Dr Andrew Cook/Gemma Price). (b) Cadaveric specimen. View from the right atrium showing structures adjacent to the oval fossa (OF). SVC superior vena cava, IVC inferior vena cava, CS coronary sinus, Ao aorta (Image courtesy of Dr Andrew Cook/Gemma Price). (c) Cadaveric specimen. View from the right atrium showing structures within the triangle of Koch. TV tricuspid valve (Image courtesy of Dr Andrew Cook/Gemma Price). (d) Cadaveric specimen. View from the left atrium (Image courtesy of Dr Andrew Cook/Gemma Price) LAA left atrial appendage, MV mitral valve
When viewed through the left atrial wall, the anatomy is less complex. Only a small portion of the anterior region of the atrial wall is true septum, the rest representing overlap and fusion of the primary atrial septum (flap valve) with the anterior atrial wall (Fig. 26.2d).
The atrial septum is a curved structure and sits at an angle within the heart relative to the anteroposterior position of the thorax – this is a simple concept but one that poses challenges for the delivery of a device delivered through a sheath positioned along the natural line of the inferior vena cava. Defects in the secundum atrial septum are variable in both size and position. Although a proportion are truly central, eccentric holes with extension to any margin can occur. Secundum ASDs are not infrequently multiple and often sit within mobile or “aneurysmal” septal tissue. All of these anatomical variables must be defined prior to the delivery of a device.
26.3 Physiology
Assuming that an ASD is of significant size, the magnitude of the shunt is determined by the relative resistance to filling of the ventricles. Right ventricular resistance is usually less than the left and therefore the overall shunt is left to right. Flow across an ASD is phasic occurring predominantly in late ventricular systole and early diastole. Abnormalities of ventricular diastolic function in either ventricle (e.g. systemic hypertension leading to left ventricular hypertrophy) will affect the direction and magnitude of the atrial shunt and are part of the reason why atrial septal shunts increase in significance with age.
26.4 Clinical Scenarios: Natural History
The vast majority of infants and children with ASDs are asymptomatic, although many have tendency to recurrent chest infections and respiratory symptoms. Very occasionally an infant will be encountered in whom an atrial shunt is responsible for failure to thrive.
During adult life, symptoms are progressive. Exercise intolerance is a common feature. Studies consistently show that with each passing decade, an increasing proportion of patients with ASDs display one or more of the characteristic sequelae of an important atrial shunt: pulmonary hypertension, atrial dysrhythmia or clinical right heart failure.
26.5 Indications for ASD Closure
Traditional indications for the closure of intracardiac shunts were based on invasive oximetry, with a shunt of >1.5:1 taken as significant. In the modern era, virtually all decisions on the significance of atrial shunts (and therefore the indications for closure) are made using non-invasive imaging prior to an attempt to close a defect.
There is an abundance of literature demonstrating that patients of any age with significant ASDs benefit from closure. In children, most units defer closure of ASDs until around the 3rd birthday at the earliest unless the clinical situation is atypical, e.g. failure to thrive or there are particularly frequent chest infections.
Although in years gone by there was an active debate about the need for ASD closure in older adults, the issue of benefit for these patients has been resolved by the publication of a number of studies. Established atrial dysrhythmia is rarely solved by closing an ASD in this age group, but shunt-related symptoms are improved, pulmonary hypertension is resolved, the right heart is usually remodelled and clinical right heart failure, if present, is easier to treat medically.
Contraindications to ASD closure include defects that are anatomically unsuited to a device such as those that are greater than 40 mm in diameter or with inadequate margins. In these cases, surgery should be offered. Very rarely, patients are encountered who are affected by both primary pulmonary hypertension and a coexistent ASD. Often these patients are younger women and almost always have clinical signs out of context with the ASD itself, e.g. cyanosis due to right-to-left shunting at atrial level. Closure of an ASD in this situation should be avoided.
26.6 Treatment Options
If an ASD is significant, then the options for closure are either surgery or a transcatheter delivered device. It is important that patients and families are thoroughly counselled about the pros and cons of both procedures so that an informed decision can be made.
26.7 Device Options
1.
Currently available self-centering devices:
Amplatzer (St Jude) septal occluder (http://health.sjm.com/amplatzer-septal-occluder): Original nitinol framed occluder available in core central diameters from 4 to 40 mm (Fig. 26.1a).
Occlutech Figulla occluder (http://www.occlutech.com): Nitinol occluder with titanium coating (antithrombotic) in core diameters from 4 to 40 mm. The left atrial disc does not have a screw or metallic protrusion (Fig. 26.1b) and the delivery system is innovative, allowing articulation of the device on the delivery wire to facilitate closure of larger or asymmetric defects (Fig. 26.1c).
Cera (Lifetech) occluder (http://www.lifetechmed.com): Titanium-coated nitinol occluder in 6–42 mm core sizes.
Cardia (http://www.cardiainc.com): The Ultrasept ASD occluder is constructed of nitinol/titanium wires covered with Ivalon sails with core sizes from 6 to 34 mm (Fig. 26.1c).
Device delivery systems: All of the devices described above have their own pre-curved delivery sheaths of varying internal diameter (depending on the size of device) to facilitate delivery of the device. They are all loaded into a short tube before being introduced into the delivery sheath. Modern self-centering devices are generally retrievable and repositionable prior to final release from the delivery cable.
2.
Non-self-centering devices:
Occlutech, Cera and St Jude (Amplatzer) all produce a variant of their self-centering nitinol mesh-based device with a thin central core designed for coverage of multiple defects. These devices give maximal coverage of the defect without the restriction of a central core. This facilitates coverage of multiple holes at the expense of stability (i.e. the potential for movement) within the septum. These devices are delivered in exactly the same manner and through the same delivery sheaths as their self-centering equivalents.
Cardia: Cardia produce an Ultrasept cribriform based on the same principle as the Cardia ASD occluder but without the central core.
Gore Septal Occluder (GSO) (http://www.goremedical.com/eu/septaloccludereu/): A newer form of occluder based on five interlocking nitinol/platinum wires covered with an ePTFE shell. The GSO has a soft “scalloped” construction that allows the device to adapt to the contours of the heart with an extremely low profile (Fig. 26.1d). The device comes pre-attached to a delivery handle, and the delivery sheath is part of the pre-assembled system. The device is advanced to the atrial septum using a monorail port at the end of the wire. Device deployment is by a simple movement of the delivery button and the device can be retracted and deployed as many times as required.
26.8 Pre-procedural Imaging
Most units rely on transthoracic echocardiography (TTE) for initial assessment. Clear evidence of right heart dilation on TTE is usually a marker of a significant atrial shunt. In children TTE can also reliably delineate the anatomy, the margins of the defect and the presence of associated abnormalities (e.g. anomalous pulmonary venous drainage or mitral valve disease). In adults, TTE sometimes does not provide the resolution to accurately define the anatomy and margins of the defect. In many cases of this sort, it is appropriate to move on to attempted closure with a careful trans-oesophageal echocardiography (TEE) assessment to check suitability for closure before catheterisation. In some cases a pre-procedural MRI can be helpful. MRI has the additional advantage of providing accurate volumetric analysis and relative pulmonary/systemic flows ratios if there is any doubt about the indication for closure. It also provides clear imaging of the pulmonary veins. Against this the spatial resolution of MRI means that imaging of the atrial septum itself is not always as accurate as with ultrasound.
An important part of the assessment of an adult patient with an ASD should be a full assessment of left ventricular function. Impaired systolic and diastolic function can be masked by an atrial shunt and closure of the defect in these patients can (rarely) precipitate pulmonary oedema. In older adults (>50 years), it is important to rule out and if necessary treat coexistent coronary abnormalities/disease.
26.9 Techniques: Step by Step
1.
Pre-procedure




Ensure appropriate patient selection.< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


