Serial echocardiographic follow-up of patients with a bicuspid aortic valve (BAV), in addition to providing assessment of valve dysfunction, can help identify those at risk of aortic complications. However, currently there is no standardized echocardiographic method for measuring the ascending aorta. We examined the echocardiograms of 45 patients with a BAV and 45 matched controls to understand the effects of the measurement location (1, 2, and 3 cm above the sinotubular junction) and the point in the cardiac cycle (end-diastole, mid-systole, and end-systole) at which the ascending aortic measurements are made. A greater length of aorta could be measured in end-systole than in end-diastole, presumably because of aortic recoil. Using the control data for comparison, we found that more dilated ascending aortas were detected by measuring 3 cm above the sinotubular junction in the patients with a BAV (56%) than at 1 cm (42%). The increases in size between 1 and 2 cm were greater than those between 2 and 3 cm. In conclusion, we propose that all transthoracic echocardiograms should include the proximal aorta at least 2 cm and preferably 3 cm above the sinotubular junction and suggest that for standardization and optimal visualization the measurements be done at end-systole in all patients.
The bicuspid aortic valve (BAV) is the most common congenital cardiac malformation, with a prevalence of 0.5% to 2.0% in the general population. Patients with a BAV often have ascending aortic (AA) dilation that, when progressive, predisposes them to complications, even when the valve function is only mildly abnormal. Many investigators have sought to characterize aortic dilation in patients with a BAV using 2-dimensional echocardiography. Standard echocardiograms, however, often include only limited views of the proximal ascending aorta and thus can miss dilation. Published methods vary and include measurement of the ascending aorta at 1 cm beyond the sinotubular junction (STJ), 2 to 3 cm beyond the STJ, at the “maximum size” of the ascending aorta, and at the “level of the pulmonary artery.” Some reports even neglected to specify the location. In addition, the measurements have often been made at different points in the cardiac cycle, with end-diastole and end-systole the most commonly used. We, therefore, aimed to understand the implications of echocardiographic measurement of the ascending aorta in patients with a BAV and controls at various locations and points in the cardiac cycle. We believe that our findings will make it clear that a standardized method for AA measurement is needed for all patients.
Methods
We selected 71 patients with a BAV who were undergoing echocardiography as a part of a prospective study of the association of BAV and AA dilation. We subsequently reviewed 204 age- and gender-matched controls with normal tricuspid aortic valves in our echocardiographic database who had undergone routine clinical echocardiography from November 2005 to December 2006 at the University of Massachusetts Medical School. Patients with hypertension, elevated cholesterol, diabetes, Marfan syndrome, previous aortic or aortic valve surgery, severe valve dysfunction, aortic coarctation, or other congenital cardiac defects were excluded (n = 87). Of the remaining 117 control patients, 45 (38%) had adequate visualization of the ascending aorta 3 cm above the STJ throughout the cardiac cycle. Of the 71 patients with a BAV, 45 (63%) had adequate visualization of the ascending aorta 3 cm above the STJ throughout the cardiac cycle. The institutional review board approved the study.
The echocardiographic studies were performed using standard commercially available equipment. All echocardiograms were read by experienced cardiologists. Stenosis was defined arbitrarily as mild if the peak velocity was 2.5 to 2.9 m/s, moderate if it was 3.0 to 4.0 m/s, and severe if it was >4.0 m/s. Aortic insufficiency was graded according to standard methods.
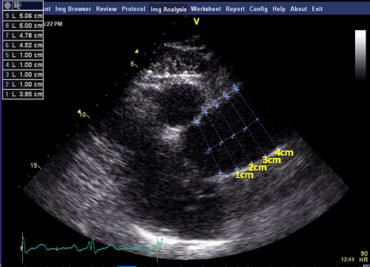
The AA diameters were measured on the left and right parasternal 2-dimensional images from the inner edge to the inner edge of the aortic lumen perpendicular to the long axis at 3 locations: 1, 2, and 3 cm from the STJ ( Figure 1 ). A fourth measurement was made at 4 cm when possible. Each set of measurements was made at 3 points in the cardiac cycle: end-diastole, mid-systole, and end-systole. One of us (AA) reviewed the images and measured the aortic diameters for all subjects. The measurements were repeated in a subset of 18 subjects in end-diastole and end-systole (108 data points). A second observer (LP) repeated measurements in a subset of 10 subjects. These data were used to assess the intra- and interobserver variabilities.
The data are expressed as the mean ± SD. The upper limit of normal for the aortic diameter was defined as the control + 2 SD at each location and was used to determine the percentage of patients with a BAV with aortic dilation.
Results
No significant differences were found between the 45 patients with BAV and the 45 control patients with respect to demographics ( Table 1 ). None of the patients with a BAV had severe aortic stenosis or insufficiency. Minimal intra- and interobserver variabilities were found in the measurement of the aortic dimensions ( Figure 2 ).
| Characteristic | BAV (n = 45) | Control (n = 45) |
|---|---|---|
| Age (years) | 47.5 ± 12.6 | 47.6 ± 12.6 |
| Men (%) | 60 | 60 |
| Height (cm) | 172 ± 11 | 173 ± 12 |
| Weight (kg) | 83 ± 23 | 88 ± 20 |
| Body surface area (m 2 ) | 1.96 ± 0.27 | 2.01 ± 0.26 |
| Heart rate (beats/min) | 70 ± 10 | 77 ± 12 |
| Systolic blood pressure (mm Hg) | 121 ± 14 | 119 ± 8 |
| Diastolic blood pressure (mm Hg) | 75 ± 10 | 76 ± 10 |
| Ejection fraction (%) | 63 ± 8 | 63 ± 4 |
| Mild aortic stenosis | 19 (42%) | 0 |
| Moderate aortic stenosis | 2 (4%) | 0 |
| Severe aortic stenosis | 0 | 0 |
| Mild aortic insufficiency | 16 (36%) | 5 (11%) |
| Moderate aortic insufficiency | 12 (27%) | 0 |
| Severe aortic insufficiency | 0 | 0 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


