Histologic diagnosis
Endomyocardial biopsy demonstrating noncaseating epithelioid granulomas
Clinical diagnosis
Suspect cardiac sarcoidosis with (a) + one other
(a) Complete right bundle branch block, AV block, ventricular tachycardia, ventricular premature beats or pathologic Q wave or ST-T changes on the electrocardiogram
(b) Abnormal wall motion, regional wall thickening, or left ventricular dilation
(c) Perfusion defect on myocardial perfusion imaging or abnormal accumulation of 67-Gallium citrate or 99mTc-PYP myocardial scintigraphy
(d) Abnormal intracardiac pressure, low cardiac output, or abnormal wall motion or reduced ejection fraction of the left ventricle
(e) On endomyocardial biopsy, interstitial fibrosis or more than moderate cellular infiltration, even if the findings are nonspecific
Cardiac magnetic resonance imaging (cMRI) and cardiac 18-flouro-deoxuyglucose positron emission tomography (FDG-cPET) are being utilized as noninvasive diagnostic tests of choice for cardiac sarcoidosis. However, both cMRI and FDG-cPET are expensive, not readily available, and are likely not the best choice for routine screening.
In this chapter, we will review screening for cardiac sarcoidosis using the more readily available clinical tools of electrocardiography, signal-averaged ECG, ambulatory monitoring, and echocardiography.
Electrocardiography
Simple, inexpensive, and readily available characterize electrocardiography (ECG). Unfortunately, due to the short time frame of sampling with ECG, many findings may be missed, especially intermittent conduction disease. Complete heart block is the most common finding in patients with clinically evident cardiac sarcoidosis. Heart block often occurs at a younger age in patients with sarcoidosis than in individuals with complete heart block due to other etiologies [4]. First-degree AV block (PR prolongation) due to atrio-ventricular nodal disease or bundle of His, and other intraventricular conduction diseases are frequently seen [5]. It is important to note that most of these issues may initially be silent but can often rapidly progress to complete heart block, marked bradycardia, and syncope. A low threshold to perform an ECG at a moment’s notice should be part of the clinical toolbox available to sarcoidosis patients without currently known cardiac involvement.
As the sarcoidosis disease process develops, granulomas can develop in almost any tissue. As they develop in the heart, substrate for arrhythmia is created in that granulomas can effectively block or redirect conduction. Since granulomas develop into scar, they do not conduct electrical impulses, and can create islands of non-conducting tissue that have the potential to develop arrhythmia. Areas of active inflammation may also create areas of automaticity leading to re-entrant dysrhythmias. Ventricular arrhythmias are the second most common presentation of cardiac sarcoidosis. This includes both sustained and non-sustained ventricular tachycardias. As many as 22 % of patients with sarcoidosis may demonstrate ventricular arrhythmia on ECG [6].
In clinical practice, and in the electrophysiology realm (see Chaps. 8, 9, and 12), atrial sarcoidosis has been described creating virtually any atrial dysrhythmia such as atrial fibrillation, atrial flutter, and atrial tachycardia.
In any person with otherwise unexplained ventricular arrhythmia, heart block, or conduction disease, a search for sarcoidosis involving the conduction system should be part of the workup.
Signal-Averaged ECG
Signal Averaged ECG (SAECG) is a simple non-invasive electrocardiographic test that detects low amplitude signals at the end of the QRS complex that are known as “late potentials” [7]. SAECG has been used clinically to identify patients at increased risk of ventricular arrhythmias [8] after myocardial infarction or with arrhythmogenic right ventricular dysplasia. In one study, SAECG was abnormal in 46 % of patients with pulmonary sarcoidosis with no clinical evidence of cardiac sarcoidosis [9]. The presence or absence of cardiac sarcoidosis was not confirmed by imaging modalities in that study.
A recent study by Schuller et al. [10] evaluated patients with suspected cardiac sarcoidosis as defined by Japanese Ministry of Health criteria and/or delayed gadolinium contrast enhancement on cardiac MRI. In this study, they found 27 of 88 patients included had cardiac sarcoidosis based on JMHW criteria with MRI. The authors found the sensitivity of SAECG detection of cardiac sarcoidosis was 52 % with a specificity of 82 %. In addition, they calculated a positive predictive value (PPV) of 0.56 and a negative predictive value (NPV) of 0.79. Within a subgroup of the 67 patients with an unfiltered QRS duration of <100 ms, they found the specificity for diagnosing cardiac sarcoidosis improves to 100 % with a reduced sensitivity of 36.8 % (Table 4.2).
Table 4.2
Criteria for abnormal signal averaged ECGs
Criteria used for establishing positive signal-averaged ECG |
|---|
1. Noise must be less than 0.3 to be interpretable |
2. Filtered QRS: ≤124 ms in males; ≤116 ms in females |
3. HFLA/LAS: ≤42 ms in males and females |
4. Root Mean Square (RMS): ≥16 in males; ≥15 in females |
Echocardiography
In sarcoidosis patients without an obvious cause for heart failure, cardiac sarcoidosis rises to the top of the differential diagnosis. Echocardiography makes it easy assess ventricular function as well as any potential valvular involvement or wall motion abnormality. In patients with other known cardiac abnormalities such as those found in the conduction system, echocardiography should be performed.
In a study by Burstow et al. echocardiographic changes were detected in 14 % of patients with systemic sarcoidosis, but in another 11 %, echocardiogram was within normal limits although there were significant clinically unexplained conduction abnormalities likely related to sarcoidosis [11].
Possible abnormalities seen on echocardiography include left ventricular cavity enlargement, septal wall thinning (particularly of the basal segment) or segmental wall motion abnormalities of the left ventricle, left ventricular aneurysm, valvular regurgitation. Also possible is mitral valve prolapse, but this is not specific to cardiac sarcoidosis involvement. Right ventricular dilatation and hypokinesis can be seen as well, particularly with extensive pulmonary involvement, and with development of pulmonary hypertension. There are several reports of hyperechoic (commonly referred to as “sparkling”) signal of the left ventricular myocardium when there is granulomatous involvement and scar formation [12] (Fig. 4.1).
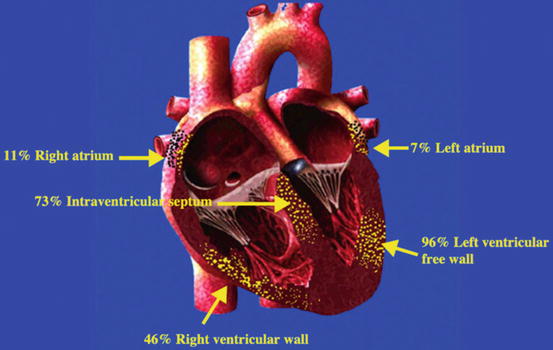

Fig. 4.1
Likely locations of cardiac sarcoidosis involvement (From Bargout and Kelly [18] with permission)
Sarcoidosis lesions of the myocardium may show up with an increased myocardial wall thickness, simulating left ventricular hypertrophy, or increased interventricular septal thickness, resembling hypertrophic cardiomyopathy.
If wall motion abnormalities are present from cardiac sarcoidosis, they usually do not follow typical coronary distributions. It is of course certainly possible to have coexistent coronary disease, and ischemia should be sought as a cause of wall motion abnormalities in appropriate individuals.
Pulmonary hypertension, described in Chap. 13, is often detected with echocardiography in patients with pulmonary hypertension. In these cases, elevated right ventricular systolic pressure in the setting of right heart dysfunction often suggests this disease process. This diagnosis must be confirmed with directly measured values via a right heart catheterization. The World Health Organization classifies this as group V pulmonary hypertension (pulmonary hypertension due to underlying systemic diseases).
Ambulatory Electrocardiographic Monitoring
Ambulatory electrocardiographic monitoring, most often known as Holter or mobile telemetry monitoring, reduces the problem of too short of a sampling period with a standard ECG. Using either 24 or 48 h monitoring, clinicians can determine not only if there is a conduction defect, but can also significantly monitor the burden of arrhythmia, ventricular ectopy, and even evaluate for atrial arrhythmias, Ambulatory ECG monitoring has proven itself as the most useful of the non-imaging modalities [2].
In a small study of 38 patients [13] with systemic sarcoidosis referred for cardiologic evaluation, 7 of 12 patients (67 %) with confirmed cardiac sarcoidosis had >100 premature ventricular contractions per day as compared to only 8 % of the 26 patients without cardiac sarcoidosis. In that study, only 5 % of 58 healthy controls had this degree of ventricular ectopy. This study demonstrated that Holter monitoring has a sensitivity of 67 % and a specificity of 80 % [13].
Many centers use Holter monitoring as part of the yearly surveillance of patients with sarcoidosis, as it represents a very inexpensive yet thorough assessment of the cardiac conduction system. It has also been the basis for many ICD implants, in that non-sustained ventricular tachycardia detected on such a device often necessitates additional workup such as electrophysiology study, advanced imaging such as cardiac MRI to determine scar burden, and more.
Sudden cardiac death (SCD) due to ventricular tachyarrhythmias or serious conduction block accounts for up to 65 % of deaths due to cardiac sarcoidosis [14]. While many patients with known systemic sarcoidosis develop symptomatic conduction disease on electrocardiogram prior to sarcoidosis-related sudden death, many patients do not. It is important to underscore the understanding that sudden cardiac death (SCD) can occur in the absence of symptoms or a previous cardiac event [15].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


