Stable Angina: Diagnosis, Risk Stratification, Medical Therapy, and Revascularization Strategies
The syndrome of angina pectoris, first described in 1768 by William Heberden, is most commonly the symptomatic result of fixed coronary artery obstruction and impaired endothelial vasomotor activity in patients with advanced coronary atherosclerosis. In stable angina, symptoms occur in a predictable and reproducible fashion during periods of physical or emotional stress, when increases in heart rate, cardiac contractility, and afterload increase myocardial oxygen requirements. Relief is usually brought on with rest or nitroglycerin. Symptom severity can be widely variable from patient to patient and is most commonly graded according to the Canadian Cardiovascular Society (CCS) scale (Table 39.1).1
TABLE
39.1 CCS Classification of Angina

From Campeau L. Letter: grading of angina pectoris. Circulation. 1976;54(3):522–523.
The epidemiology of coronary artery disease (CAD) and stable angina has changed in the United States. As a result of improved medical and revascularization strategies, the myocardial infarction (MI) survival rate has improved, thus allowing patients to live longer with more chronic manifestations of CAD. From 1997 to 2007, the annual death rate due to CAD decreased by 26.3%, with more than 80% of deaths in patients 65 years of age or older.2 With the aging of the population, there has been an increase in the prevalence of CAD, with current estimates showing over 16 million Americans living with stable CAD. This represents approximately 7% of the U.S. population over 20 years of age.2
Despite these substantial improvements, CAD remains the number one killer of both men and women in the United States, causing about one in six deaths in 2007.2 Stable angina pectoris remains among the most common initial clinical manifestations of CAD. Of the 16 million Americans with known CAD, an estimated 9 million live with chronic stable angina, a number that is expected to increase as the population continues to age.2 The presence of symptomatic CAD affects quality of life negatively. It imparts significant morbidity and mortality, with estimates of just over 10% annual incidence of either a nonfatal MI or coronary death in patients presenting with stable angina.3 While most of the early data were in men, more recent data suggest an equally poor outcome in women presenting with angina.4
DEFINITION OF STABLE ANGINA
Angina is defined as the sensation of chest discomfort that occurs in the setting of myocardial ischemia in the absence of myonecrosis.5 It is traditionally described in terms of its clinical setting, characteristics (quality, location, radiation, etc.), precipitating or alleviating factors, and time course to cessation. The classic description is of substernal pressure or heaviness that can radiate to the left arm, jaw, neck, or shoulders. Chronic stable angina usually occurs due to fixed coronary obstructions of ≥50% of the diameter of the left main trunk or ≥70% of the diameter of one of the other epicardial coronary arteries and is a demand phenomenon.5 The pain is predictable, occurring with physical activity at a known threshold or related to emotional stress. Most often, it is relieved quickly with rest and does not last longer than 10 minutes.5 Typical stable angina meets all three of the following criteria: substernal location with the characteristic quality and duration, provoked by exertion or emotional stress, and relieved by rest or nitroglycerin.6
Unfortunately, classic symptoms or triggers are not present in all patients, most notably diabetics, women, and the elderly. This is referred to as atypical angina and can sometimes lead to later diagnoses and poorer outcomes.5 Atypical angina meets two of the three characteristics listed above. Patients who do not experience any chest discomfort can still be diagnosed as having stable angina. Diabetics and the elderly are more likely to experience anginal equivalents, such as dyspnea, diaphoresis, fatigue, nausea, light-headedness, altered sensorium, or syncope.5 It has been theorized that the lack of chest discomfort is related to altered pain perception or autonomic neuropathy. In its most ominous form, the ischemia can be completely silent.5 Finally, noncardiac chest pain is defined as pain that meets one or zero of the characteristics of typical angina.6
PATHOPHYSIOLOGY OF STABLE ANGINA
Simply put, angina occurs as the result of an imbalance between myocardial oxygen/substrate supply and demand.5 Conditions that alter oxygen or other substrate (glucose and free fatty acids) supply can produce angina even in spite of a normal demand state. Conversely, with exercise or emotional stress, myocardial demand is increased due to increased heart rate, blood pressure, and left ventricular contractility in relation to a sympathetic nervous response. With significant CAD, the oxygen/substrate supply is fixed due to the inability of coronary autoregulation to increase coronary blood flow due to the fixed stenosis. Oxygen extraction in the coronary arteries is already at a maximum at baseline, so increased extraction cannot satisfy the imbalance between supply and demand. Thus, the patient experiences angina in these states of increased demand.5
DIAGNOSTIC TESTING/RISK STRATIFICATION
Evaluation of the patient with symptoms suspicious for CAD begins with assessing for the presence of CAD historical predictors, such as pain character and setting, age, gender, diabetes mellitus, smoking, hypertension, and hyperlipidemia.7 This allows one to establish the pretest likelihood of disease and helps determine which, if any, diagnostic testing is needed. Each patient presenting with suspected stable angina should have a thorough history and physical examination as well as an electrocardiogram (ECG) (American College of Cardiology/American Heart Association [ACC/AHA] Class I recommendation).6 There are certain features in this initial workup that can indicate a high, intermediate, or low likelihood of CAD and help to guide further evaluation (Table 39.2). In those with a significant suspicion for CAD, the spectrum of risk is broad and warrants different evaluation and treatment strategies for different levels of risk. Conversely, patients with low-risk features may warrant a more conservative and less invasive evaluation and treatment course. However, stratifying patients in this manner is not fail-safe; it is still possible to have obstructive CAD but with low-risk symptoms or features. The only way to completely rule out the presence of CAD is with a coronary angiogram.5
TABLE
39.2 Clinical Features and Likelihood of CAD in Patients Presenting with Angina
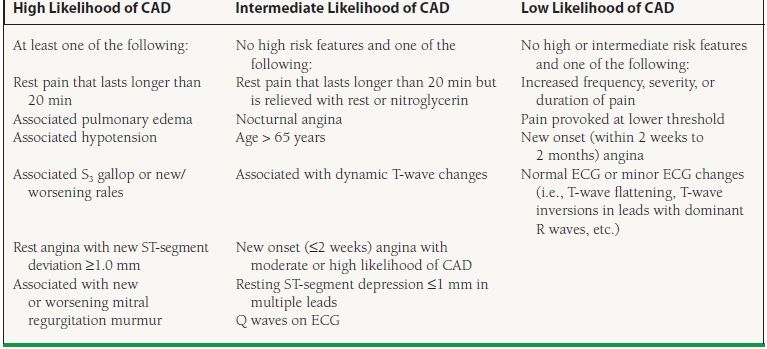
From Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina). Circulation. 2GG3;1G7(1):149–158, with permission.
Electrocardiography
Although the 12-lead ECG is normal in more than half of patients with chronic stable angina, an ECG is a readily available first-line test that provides both diagnostic and prognostic information (ACC/AHA Class I recommendation).6 A normal ECG at the time of diagnosis is associated with a favorable long-term prognosis, whereas abnormalities such as left ventricular hypertrophy, Q waves suggestive of prior MI, and persistent ST-segment depression identify patients at higher risk of future adverse events.6,8 In the half of patients with a normal baseline ECG, one obtained during an episode of pain will be abnormal in another 50% of patients. Evidence of ST-segment deviation signals a high likelihood of CAD and has a more unfavorable prognosis.6 Patients with ECG abnormalities at baseline can have “pseudonormalization” of these during a pain episode; this also indicates a high likelihood of CAD.6 Depending on the ECG and the underlying likelihood of CAD, a decision is made to either pursue further noninvasive testing or proceed straight to a coronary angiogram.
Exercise ECG Testing
One of the oldest and most widely used noninvasive tests for CAD is the treadmill exercise ECG, because of its widespread availability, low cost, and ease of performance.5 This is generally the first test selected to evaluate patients with an intermediate likelihood of CAD and a normal baseline ECG who are able to exercise (ACC/AHA Class I recommendation).6 The absolute contraindications to exercise ECG testing include hemodynamically significant arrhythmias, within the first 48 hours of MI, symptomatic heart failure, symptomatic or severe aortic stenosis, myocarditis, acute aortic dissection, and acute pulmonary embolus. There are also ECG abnormalities that are not suited for exercise ECG testing, as they make interpretation of the exercise ECG impossible. These include Wolff–Parkinson–White syndrome, paced ventricular rhythms, >1 mm resting ST-segment depression, and complete left bundle branch block (ACC/AHA Class III recommendation).6
In a large meta-analysis of exercise stress ECG, the mean sensitivity and specificity for detecting angiographically significant CAD were 68% and 77%, respectively.6 Although exercise ECG is less sensitive than stress tests performed with imaging modalities, particularly in women, it remains the primary noninvasive tool for both the diagnosis and risk stratification of patients with suspected CAD and interpretable ECGs. The ACC/AHA guidelines recommend that, unless cardiac catheterization is more urgently indicated, symptomatic patients with suspected or known CAD should be considered for exercise ECG testing to assess the risk of future cardiac events and the possible need for angiography.6 As a diagnostic tool, exercise ECG testing is most useful in patients with stable chest pain syndromes and an intermediate risk of CAD (ACC/AHA Class I recommendation). In those with a low or high pretest probability of CAD, exercise ECG has a Class IIb recommendation.6 As a prognostic tool, it can help to identify patients with extensive atherosclerosis who would benefit from coronary angiography and possible revascularization.
In addition to the ECG portion, there are other variables that contribute to the interpretation of the test. The usual definition for a positive exercise ECG test is 1 mm or more of ST-segment elevation or horizontal or downsloping ST-segment depression at a point 60 to 80 milliseconds after the QRS complex during exercise or recovery.6 Additionally, symptoms, exercise capacity, and hemodynamic and rhythm response to exercise should be considered.6 The most important prognostic variables measured during exercise testing are exercise capacity, typically expressed in metabolic equivalents of task (METs), and exercise-induced ischemic ST-segment changes. The Duke Treadmill Score (DTS) integrates these two objective variables with the subjective presence or absence of anginal symptoms to generate a risk score that separates patients into high-, moderate-, and low-risk subsets (5%, 1.25%, and 0.25% annual mortality rates, respectively) (Table 39.3).9 Patients with high-risk DTSs frequently have left main or three-vessel CAD that would benefit from revascularization, and these patients should be referred for coronary angiography. Low-risk patients, on the other hand, have an excellent prognosis that is unlikely to improve with further evaluation or revascularization and thus can be treated safely with medical therapy.
TABLE
39.3 Duke Treadmill Score
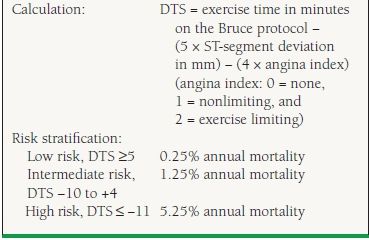
From Mark DB, Shaw L, Harrell FE Jr, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325(12):849–853, with permission from the Massachusetts Medical Society.
Most patients undergoing a diagnostic evaluation for stable angina do not require echocardiography. More specifically, in patients with a normal ECG, no history of prior MI, and no clinical signs or symptoms of heart failure, valvular disease, or hypertrophic cardiomyopathy, it is currently contraindicated to obtain an echocardiogram (ACC/AHA Class III recommendation).6 An exception is when there is a murmur suspicious for aortic stenosis or hypertrophic cardiomyopathy on physical exam or when the echocardiogram can be obtained during or within 30 minutes of presentation with chest pain to evaluate for regional wall motion abnormalities (ACC/AHA Class I recommendation).6 In this setting, regional wall motion abnormalities have a positive predictive value for ischemia of approximately 50%, whereas normal studies identify patients at low risk for an acute infarction.6,10
Stress Testing with Nuclear or Echocardiographic Imaging
Although stress imaging modalities have greater diagnostic accuracy than exercise electrocardiography, the increased cost of these tests precludes their routine use in all patients with suspected CAD. Most commonly, nuclear (single positron emission computed tomography [SPECT] or positron emission tomography [PET]) or echocardiographic stress imaging is reserved as first-line testing in patients with abnormal baseline ECGs (i.e., pre-excitation, resting ST-segment depression ≥1 mm) or with symptoms and history of prior revascularization (percutaneous coronary intervention [PCI] or coronary artery bypass grafting [CABG]) (ACC/AHA Class I recommendation).6 In patients with either paced ventricular rhythms or left bundle branch block, pharmacologic stress myocardial perfusion imaging is preferred over stress echocardiography due to the difficulty with interpreting echocardiographic wall motion in these conditions (ACC/AHA Class I recommendation).6 For patients who are unable to exercise, pharmacologic stress myocardial perfusion imaging or dobutamine stress echocardiography is equally preferred (ACC/AHA Class I recommendation).6 For a summary of the ACC/AHA recommendations, see Table 39.4.
TABLE
39.4 ACC/AHA Recommendations for Exercise ECG Testing and Stress Imaging Studies in Stable Angina Pectoris
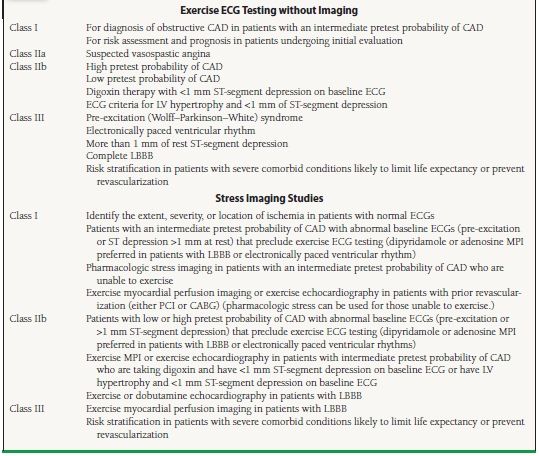
CABG, coronary artery bypass grafting; CAD, coronary artery disease; ECG, electrocardiogram; LBBB, left bundle branch block; LV, left ventricular; MPI, myocardial perfusion imaging; PCI, percutaneous coronary intervention.
From Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina). Circulation. 2003;107(1):149–158, with permission.
As mentioned, stress myocardial perfusion imaging has a higher sensitivity for the diagnosis of CAD than exercise ECG in patients with intermediate risk. The data vary depending on the population studied, but generally the sensitivity is accepted to be around 90% for myocardial perfusion imaging.5 Similar numbers are seen with exercise stress echocardiography (sensitivity around 85%) and dobutamine echocardiography (sensitivity around 82%).6 The choice of test depends on both patient characteristics and local expertise in performing the different imaging modalities.
There are some special populations or situations in which exercise stress imaging should be considered over exercise ECG. Women have an overall lower prevalence of CAD than men and thus have a lower pretest probability of disease, making false-positive exercise ECGs more common. The higher sensitivity of stress imaging, therefore, could theoretically improve on the positive predictive value of the exercise ECG.6 Additionally, elderly patients are oftentimes less able to exercise due to comorbid medical conditions or deconditioning. Therefore, pharmacologic stress imaging may be the test of choice in this population.6 As with exercise electrocardiography, stress imaging results can also provide prognostic information, separating patients who are appropriate for medical therapy (low risk, ≤1% annual mortality) from those who may benefit from further angiographic evaluation and possible revascularization (intermediate risk, 1% to 3%; high risk, ≥3% annual mortality) (Table 39.5).11
TABLE
39.5 Risk Stratification Based on Findings of Noninvasive Testing
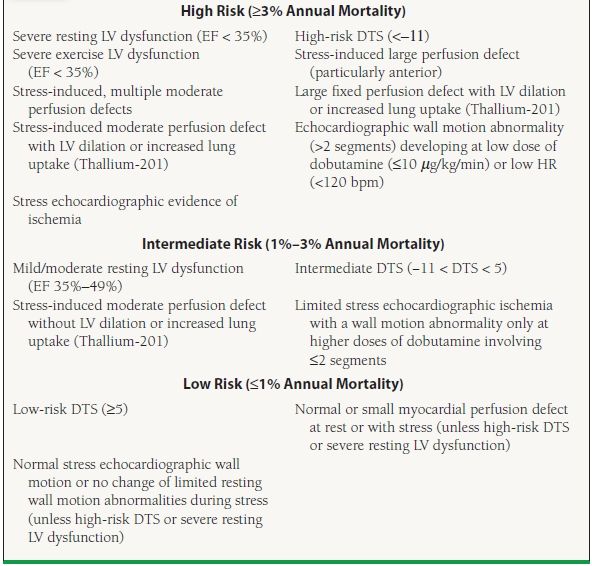
DTS, Duke Treadmill score; EF, ejection fraction; HR, heart rate; LV, left ventricular.
From Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina). Circulation. 2003;107(1):149–158, with permission.
Coronary Angiography
Coronary angiography remains the gold standard diagnostic test for CAD. Additionally, coronary angiography is able to provide anatomic definition of disease extent and severity as well as prognostic information, identifying those patients who would achieve survival benefits related to surgical revascularization. Specifically, from the early studies of CABG, patients with severe left main trunk stenosis, three-vessel disease, and two-vessel disease involving the proximal left anterior descending (LAD) coronary artery are known to derive a survival benefit with bypass surgery over medical therapy.12
In general, coronary angiography is performed in patients with stable chest pain syndromes when noninvasive tests are inconclusive or cannot be performed, when clinical evaluation or noninvasive testing suggests high-risk features (see Table 39.5), and when symptoms persist despite appropriate medical therapy. Less commonly, diagnostic coronary angiography is recommended for patients in whom coronary artery spasm is suspected, those with occupations that necessitate a definitive diagnosis (e.g., pilots, police, professional athletes), and for survivors of sudden cardiac death.13 For the list of ACC/AHA recommendations related to coronary angiography, see Table 39.6.
TABLE
39.6 ACC/AHA Recommendations for Coronary Angiography in Stable Angina Pectoris
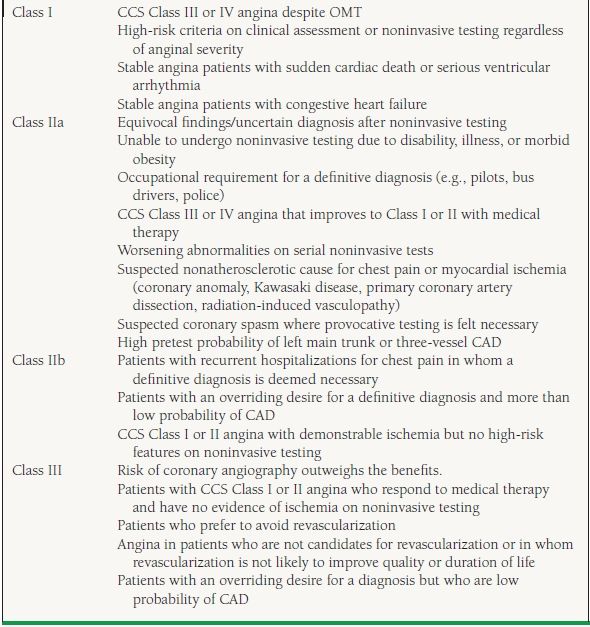
CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; OMT, optimal medical therapy.
From Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation. 1999;99(17):2345–2357, with permission.
For patients determined to be at low risk of adverse events, medical therapy alone is usually sufficient and may be superior to an invasive approach. For the moderate- or high-risk subsets, there is older evidence from randomized trials that medical therapy coupled with surgical revascularization improves long-term survival over medical therapy alone.12 Therefore, the judicious use of noninvasive and invasive studies can help establish the diagnosis of CAD while simultaneously performing the critical risk stratification that is essential in determining the appropriate risk-reducing treatment strategies, from medical therapy alone to medical therapy plus revascularization.
Conclusion
Chest pain is the most common initial presenting symptom for patients diagnosed with CAD. Although coronary angiography provides powerful prognostic information and remains the gold standard for diagnosis, noninvasive tests are often more appropriate initial tools for patients with low or intermediate clinical predictors of CAD. Even for patients with a high pretest probability of CAD, noninvasive testing can be a useful prognostic tool that allows for selection of patients who warrant further invasive evaluation.
MEDICAL TREATMENT FOR STABLE ANGINA
There are two primary goals of medical treatment for chronic stable angina: prevent MI and cardiovascular death as well as improve quality of life by decreasing anginal symptoms and occurrence of ischemia.6 However, the process of atherosclerosis cannot be reversed by medications or by revascularization procedures. Lifestyle changes can and do influence the disease course and are most often underutilized in the treatment of patients with stable CAD.14 Lifestyle changes are inexpensive, readily available, and very effective but require a motivated patient as well as support and emphasis from the physician. Lifestyle changes should be implemented first-line and should be complementary to medical therapy.
PCIs became increasingly more common in the late 1990s and early 2000s due to an overall favorable effect on reducing anginal symptoms in numerous studies of PCI versus medical therapy in CAD.15–23 The early meta-analyses of these studies did not show a reduction in mortality or MI incidence compared to medical therapy alone in these patients,24,25 even though a more recent meta-analysis did show a possible improvement in overall mortality with PCI.26 As mentioned previously, CABG does have clear-cut long-term survival benefits versus medical therapy alone, but only in a minority of patients with high-risk angiographic features.12 An initial trial of medical therapy, therefore, remains the mainstay of treatment for the majority of patients with chronic, stable CAD. This is achieved through a combination of therapies that target both ischemic symptoms and modifiable risk factors known to aggravate angina and cardiovascular disease. Medications known to reduce the risk of MI and death receive the highest priority. Medications aimed at improving quality of life by reducing the frequency and severity of anginal episodes serve as important supplementary therapies.
Medical Therapy to Improve Survival
As discussed in the next section, the symptoms of angina may be effectively reduced with the use of standard antianginal medications (e.g., beta-blockers, nitrates, and calcium channel blockers [CCBs]), but these therapies have not been shown to improve survival or reduce MI incidence in patients with otherwise uncomplicated stable angina. Therefore, the management of patients with stable CAD has evolved to include a set of standard therapies directed specifically at reducing adverse clinical outcomes such as death and MI.
The benefit of aspirin in a broad spectrum of patients with both stable and unstable atherosclerotic syndromes has been well established for decades.27 Aspirin exerts its antiplatelet effects by inhibiting cyclooxygenase, thus preventing the release of the prothrombotic platelet-aggregant thromboxane A2. Although it does not improve symptoms, clinical trials of aspirin in patients with chronic stable angina have demonstrated risk reductions for adverse cardiac events that are of a magnitude similar to that seen in patients with unstable coronary syndromes.27 In the Swedish Angina Pectoris Aspirin Trial,28 the largest randomized trial of aspirin therapy for chronic stable angina, the addition of 75 mg of aspirin to sotalol resulted in a 34% reduction in the primary composite endpoint of MI and sudden death and a 22% to 32% reduction in the measured secondary vascular endpoints (vascular death, all-cause mortality, and stroke). A similar 33% reduction in adverse cardiovascular events (vascular death, stroke, and MI) was demonstrated among 2,920 patients with stable angina included in a meta-analysis performed by the Antithrombotic Trialists’ Collaboration.27 Therefore, aspirin, administered at 75 to 162 mg daily, is first-line therapy in all chronic CAD patients (ACC/AHA Class I recommendation).29
Thienopyridines are a second class of beneficial antiplatelet agents that exert their effects by irreversibly and selectively inhibiting the binding of adenosine diphosphate (ADP) to receptors on the platelet surface, thus preventing platelet activation. Without platelet activation, the glycoprotein IIb/IIIa receptor is unable to undergo a conformational change, which then makes it unable to bind fibrinogen or von Willebrand factor. In this manner, platelet aggregation is inhibited. There are currently three drugs in this class on the market: ticlopidine, clopidogrel, and prasugrel. The oldest, ticlopidine, initially showed benefit in several atherosclerotic processes including post-PCI,30–34 unstable angina,35 and peripheral arterial disease.36–38 However, its widespread use was limited by its side effect profile, which included a risk of neutropenia as well as thrombotic thrombocytopenic purpura.
Due to its more favorable side effect profile and reduction in cardiovascular events, clopidogrel has become the thienopyridine of choice in combination with aspirin for acute coronary syndromes,39 ST-segment elevation MI,40,41 and post-PCI.42,43 However, no study has specifically addressed its effect in patients with stable angina.6 In the Clopidogrel versus Aspirin in Patients at Risk for Ischemic Events (CAPRIE) trial,44 clopidogrel appeared to be more effective than aspirin, with an overall 8.7% reduction in the combined primary endpoint (MI, vascular death, or ischemic stroke), in high-risk CAD patients (i.e., those with recent MI or stroke or with symptomatic peripheral arterial disease). To date, the only setting in which clopidogrel has not been shown to improve cardiovascular outcomes is long-term primary or secondary prevention in patients with established atherosclerosis or multiple risk factors.45
Given the limited data for clopidogrel in stable coronary syndromes, it remains an ACC/AHA Class IIa recommendation as a replacement for aspirin in patients with a contraindication.6
Prasugrel, the newest member of this class, has been shown to be a more potent antiplatelet agent than clopidog-rel46 and has been shown to be more effective in reducing cardiovascular endpoints (cardiovascular death, nonfatal MI, or nonfatal stroke) in patients with acute coronary syndromes.47 However, prasugrel has a higher risk of major bleeding and to date has not been studied in stable CAD.
Lipid-Lowering Therapy
Lipid management has been guided for decades by the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP). All NCEP ATP reports have targeted low-density lipoprotein cholesterol (LDL-C) as the driving risk factor for CAD.48 However, prior to the third report of NCEP, most of the evidence for LDL-C lowering was based on trials of lipid-lowering agents other than HMG-CoA reductase inhibitors (statins), such as bile acid sequestrants, fibric acid derivatives, and niacin.6,48 In 2001, the third report of the NCEP ATP was published,49 which reviewed the data from several randomized trials of statin therapy in CAD. With these guidelines, a treatment algorithm for LDL-C was established that focused on statins as first-line agents for all patients with stable CAD. In 2004, some revisions to those recommendations were required based on the results of several newer randomized trials not included in the original guidelines.48
In general, statins lower LDL-C, total cholesterol, and triglycerides (TG), while raising high-density lipoprotein cholesterol (HDL-C), all of which are favorable in cardiovascular disease. In aggregate, the randomized trials and meta-analyses of statins in primary and secondary prevention show reductions in MI, stroke, and cardiovascular death by about one-third each, as well as a reduction in total mortality by about one-fifth.50 The ATP III algorithm categorized patients into three risk categories: (a) established coronary heart disease (CHD) or CHD risk equivalents, (b) two or more CHD risk factors, or (c) 0 to 1 CHD risk factor.48 High risk was defined as those with CHD or CHD risk equivalents (known noncoronary atherosclerotic vascular disease, diabetes, or two or more CHD risk factors with 10-year risk for CHD > 20%).49 According to the original NCEP ATP III report49 and the subsequent revisions based on newer trial data,48 statin therapy and therapeutic lifestyle changes are indicated for all stable CAD patients with an LDL-C ≥100 mg/dL, with a goal of LDL-C < 100 mg/dL (ACC/AHA Class I recommendation).29 For patients deemed high risk, there is the optional goal of treating to a more aggressive LDL-C < 70 mg/dL or with a high-dose statin (ACC/AHA Class IIa recommendation).29 If on-treatment LDL-C is ≥100 mg/dL, lipid-lowering therapy should be intensified (ACC/AHA Class I recommendation).29 If the LDL-C is 70 to 100 mg/dL at baseline, it is reasonable to treat to an LDL-C <70 mg/dL (ACC/AHA Class IIa recommendation).29 These are more aggressive goals than those set forth in the original NCEP ATP III report.
The benefit of a more aggressive lipid-lowering strategy in patients with stable CAD was definitively established by the Treating to New Targets (TNT) trial,51 among others. In this study, aggressive cholesterol reduction with 80 mg of atorvastatin daily (mean LDL = 77 mg/dL) produced an absolute 2.2% reduction in major adverse cardiovascular events (8.7% vs. 10.9%, p < 0.001) compared to the more conventional 10 mg of atorvastatin daily (mean LDL = 101 mg/dL). The results of TNT, in addition to data from other trials such as Pravastatin or Atorvastatin Evaluation and Infection Therapy—Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22),52 were the impetus for the addition of the optional LDL-C goal of <70 mg/dL in the revision of the NCEP ATP III recommendations.48 PROVE-IT TIMI 22 showed similar benefits of very aggressive LDL-C reduction in patients with acute coronary syndromes.52
In addition to improving outcomes, nuclear studies have demonstrated that statin therapy improves myocardial perfusion and reduces ischemia on ambulatory ECG monitoring in stable angina patients with both high and normal serum cholesterol levels.53 In patients with medically refractory angina that is not amenable to revascularization, aggressive lipid reduction with 80 mg daily of atorvastatin (LDL goal < 77 mg/dL) has been shown to reduce symptoms of angina and decrease myocardial ischemic segments measured by dobutamine echocardiography when compared to more conventional therapy (LDL goal < 116 mg/dL).54
In addition to LDL-C, the 2007 updated chronic angina ACC/AHA guidelines focused more heavily on other lipid parameters. If TG are 200 to 499 mg/dL, non-HDL-C (calculated as total cholesterol minus HDL-C) should be <130 mg/dL (ACC/AHA Class I recommendation) and further reduction to <100 mg/dL was optional (ACC/AHA Class IIa recommendation).29 Therapeutic options to reduce non-HDL-C are niacin or fibrate therapy in combination with statin therapy (ACC/AHA Class IIa recommendation).29 If using a fibrate in combination with a statin, the statin dose should be kept in the lower range given the high risk for associated myopathy with the combination. If, however, the TG level is >500 mg/dL, this level should be reduced in order to prevent the development of pancreatitis; this should be done prior to the initiation of statin therapy. The therapeutic options are fibrates or niacin with a goal of non-HDL-C <130 mg/dL (ACC/AHA Class I recommendation).29
Renin–Angiotensin–Aldosterone System Blockade
The benefit of angiotensin-converting enzyme (ACE) inhibition in patients with diabetes and impaired left ventricular systolic function has been firmly established by multiple large-scale clinical trials that have consistently demonstrated a reduction in adverse clinical events.6 ACE inhibitors are therefore recommended as first-line therapy indefinitely in all patients with CAD who have impaired left ventricular systolic function (left ventricular ejection fraction ≤40%) or in those with concomitant diabetes, hypertension, or chronic kidney disease (ACC/AHA Class I recommendation).29 In patients with stable CAD and preserved left ventricular function, the data have been less consistent. Although both the Heart Outcomes Prevention Evaluation (HOPE)55 and EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease (EUROPA)56 trials demonstrated decreased mortality with ACE inhibition (ramipril and perindopril, respectively) in stable CAD patients with preserved left ventricular function, a similar population of patients in the Prevention of Events with Angiotensin Converting Enzyme inhibition (PEACE)57 trial failed to benefit with trandolapril. In light of this data, ACE inhibitors are given a Class IIa recommendation in the most recent ACC/AHA guidelines for patients with CAD and mildly reduced or normal left ventricular function.29
Most of the data for the substitution of angiotensin receptor blockers (ARBs) for those intolerant to ACE inhibitors come from trials in systolic heart failure. For instance, in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM)-alternative study,58 the patients with left ventricular systolic dysfunction treated with candesartan had improvement in primary outcomes (cardiovascular mortality and admission for heart failure) over placebo. Additionally, when MI and stroke were added in a composite secondary outcome, there were reductions in secondary outcomes as well with candesartan. Notably, the population in CHARM-alternative had a medical history consistent with a stable CAD population: approximately 60% with history of MI, approximately 20% with current angina, and 15% to 25% with history of either PCI or CABG.58 In a separate arm, CHARM-added,59 patients with left ventricular ejection fraction ≤40% and already receiving an ACE inhibitor were randomized to additional candesartan or placebo. Again there was reduction in the primary composite endpoint (cardiovascular death and heart failure admission). Similarly, when MI and stroke were incorporated into the secondary endpoints, there was a significant reduction in those as well with candesartan.59 Based on these data, ARBs are recommended for patients who have hypertension, have indications for but are intolerant of ACE inhibitors, have heart failure, or have had an MI with left ventricular ejection fraction ≤40% (ACC/AHA Class I recommendation).29 Additionally, ARBs may be considered in combination with ACE inhibitors for heart failure due to left ventricular systolic dysfunction (ACC/AHA Class IIb recommendation).29
Aldosterone blockers, such as spironolactone and eplerenone, are also ACC/AHA Class I recommendations in post-MI patients without significant renal dysfunction or hyperkalemia who are already receiving therapeutic doses of an ACE inhibitor and a beta-blocker, have a left ventricular ejection fraction ≤40%, and have either diabetes or heart failure.29 This recommendation was based primarily on data in left ventricular systolic dysfunction, with spironolactone from the Randomized ALdactone Evaluation Study (RALES)60 and eplerenone in the Eplerenone Post-acute myocardial infarction Heart failure Efficacy and SUrvival Study (EPHESUS).61
Symptomatic Medical Therapies: Antianginals
The primary goal of antianginal therapy is to reduce coronary ischemia, thereby improving exercise capacity and overall quality of life.6 The currently available antianginal medications work to counteract the hemodynamic effects of flow-limiting coronary stenoses by reducing myocardial oxygen requirements and/or by promoting coronary vasodilation. Each is an effective therapy for symptom relief alone but they are often used in combination for more complete symptom control. However, unlike the therapies in the previous section, none have been shown to reduce death or MI in patients with otherwise uncomplicated stable angina pectoris.
Beta-Blockers
Beta-blockers function by competitively inhibiting the physiologic actions of catecholamines on the beta (β) receptors in the heart and vasculature. The resulting decreases in heart rate, arterial blood pressure, and myocardial contractility substantially reduce myocardial oxygen demand. Their functional benefits in stable angina have been most clearly linked to the lowering of the heart rate–blood pressure product (double product) and were definitively demonstrated by exercise studies in which beta-blocked patients experienced a delay or avoidance of ischemia onset with activity compared to baseline.62,63 By convention, the dose of beta-blocker is therefore adjusted to lower the double product, with a goal heart rate of 55 to 60 beats/min (bpm) and exercise heart rate response <75% of the rate that precipitated ischemia on stress testing.14
Although nonselective beta-blockers are effective in stable angina, the majority of the antianginal effects of beta-blockers are related to their effects at the β1 receptor, thus ß1 selective agents may be preferred. Some beta-blockers are partial agonists and are said to have intrinsic sympathomimetic activity; these are generally avoided in stable angina.14 Several of the beta-blockers (i.e., carvedilol, labetalol.), in addition to their nonselective beta-blockade, also block catecholamines at the alpha (α) receptor (specifically α1) and are profound vasodilators.14 Additionally, some betablockers are said to have antiarrhythmic and other advantageous effects. Therefore, the choice of agent should be tailored to the specific needs of the patient, and the provider should be familiar with several different agents. For a list of common beta-blockers used in stable angina, see Table 39.7.
TABLE
39.7 Beta-Blockers Commonly Used to Treat Stable Angina Pectoris
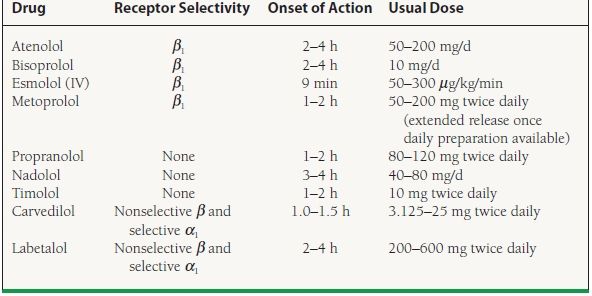
α, alpha; β, beta; IV, intravenous.



