In recent years, numerous studies have focused on the use of soluble ST2 (sST2) as a clinical biomarker for cardiovascular disease. However, much preclinical data points to involvement of the ST2 pathway in inflammation, and specifically in pulmonary inflammation. This report summarizes the current body of clinical data suggesting the potential role of the ST2 pathway in clinical disease, including evidence that sST2 could be a useful biomarker in both allergic and nonallergic pulmonary disease.
The study of the clinical utility of soluble suppression of tumorigenicity 2 (sST2) has recently focused on its promise as a biomarker in cardiovascular disease. This focus has relied on strong preclinical data, including a variety of in vitro and animal studies. For example, isolated preparations of cardiomyocytes upregulate sST2 expression under conditions of myocardial strain, and sST2 pathway abnormalities are associated with myocardial fibrosis and dysfunction in animal models. These data have been extended into the clinical setting and reinforced by studies showing the clinical utility of this biomarker for evaluation in patients with heart failure (HF). Indeed, in patients with myocardial infarction, elevated sST2 concentrations are associated with increased risk of HF events and mortality, whereas in patients with HF, sST2 concentrations are among the most powerful biomarker predictors of death or hospitalization.
In addition to the evidence suggesting a useful physiological and, by extension, clinical role for sST2 in cardiovascular disease, a large body of evidence indicates a physiological role in inflammatory disease, which logically extends beyond the cardiovascular system. ST2 is an interleukin-1 receptor family member that exists in both transmembrane and soluble isoforms. The functional ligand for ST2 is the cytokine interleukin-33 (IL-33), which is an important mediator of inflammation and immunity in several disease states. For example, ST2 may play an important role in lung inflammation, as evidenced by experiments done by Oshikawa et al using murine alveolar cell lines. In these studies, inflammatory stimuli increased endogenous ST2 production, and pretreatment of alveolar cells with sST2 significantly reduced production of cytokines.
Other studies have shown that serum sST2 concentrations are prognostically elevated in patients with disorders including asthma, idiopathic pulmonary fibrosis, severe sepsis, and trauma. In patients with acute dyspnea due to a wide mixture of pulmonary causes, Martinez-Rumayor et al reported that concentrations of sST2 were elevated in many patients with pulmonary diseases, and in this setting, the biomarker predicted risk for death. Furthermore, Hoogerwerf et al showed that sST2 concentrations were associated with disease severity and mortality in severe sepsis. Taken together, these data suggest that sST2 plays an important role in the pathophysiology of inflammatory conditions. Given the importance of inflammation in various respiratory diseases, there has been much recent interest in exploring the potential clinical utility of blood plasma concentration of sST2 as a biomarker in pulmonary medicine. Thus far, this interest has led to clinical studies in 2 broad categories: the characteristics of sST2 in allergic respiratory diseases and in respiratory diseases that involve nonallergic inflammation.
Importantly, many of the studies discussed in the following were performed using research-use-only versions of the sST2 assay, which may have different performance characteristics of the more highly sensitive Presage ST2 assay. In this regard, it is important to recognize potential differences in results if alternative assays were used to measure the biomarker.
ST2 in Allergic Respiratory Disease
Acute eosinophilic pneumonia
Of the first clinical reports of ST2 in a pulmonary condition, Oshikawa et al described the case of a patient who had elevated sST2 concentrations in both serum and bronchoalveolar lavage fluid. These levels then sequentially decreased by roughly 3-fold as the patient’s disease resolved. Since this report to our knowledge, there have not been further published reports of characteristics of sST2 in patients with this uncommon condition.
Asthma
Much interest has recently focused on the role of the IL-33/ST2 pathway in asthma and respiratory allergy with studies suggesting important regulatory potential for these factors. Some studies have advanced the direction of these findings and attempted to evaluate the clinical utility of sST2 in humans.
Oshikawa et al reported in 2001 that serum sST2 was elevated in patients with chronic asthma compared with healthy controls (p = 0.04). In patients with asthma, sST2 was differentially elevated in patients with exacerbation symptoms and was inversely correlated with peak expiratory flow ( r = −0.634, p = 0.004). Ali et al showed in that one polymorphism of the ST2 gene occurred more frequently in children with asthma than control children (p = 0.03) and that serum sST2 levels were significantly higher during acute asthma exacerbations and decreased after exacerbations: 0.29 ng/ml (95% confidence interval [CI] 0.23 to 0.36) and 0.14 ng/ml (95% CI 0.12 to 0.17), respectively (p = 0.001).
In a cohort of 82 patients presenting to an emergency department with dyspnea, patients who were found to have dyspnea due to noncardiac causes (predominantly COPD and asthma) had higher sST2 levels than patients with cardiac dyspnea, and higher levels were associated with worse outcome (difference = 195 ng/ml, 95% CI 48 to 342 ng/ml, p = 0.006). In a group of 37 children with asthma, both sST2 and IL-33 serum and sputum levels were significantly higher than those of healthy controls (p = 0.0001) and were correlated with disease activity ( r = 0.772, p = 0.0001). In addition, a study of the sST2 ligand IL-33 showed that serum IL-33 levels were associated with numerous markers of asthma severity including forced expiratory volume in 1 second, bronchial basement membrane thickness, and collagen production.
In contrast, in the Framingham Heart Study of normal subjects, Coglianese et al did not find any link between sST2 values (measured using the Presage ST2 assay) and either incident or prevalent asthma; no link between sST2 and pulmonary function testing was found either.
Taken together, these data show associations between the IL-33/ST2 system and disease activity in asthma. As yet, no study of sufficient size and quality exists to validate the role of sST2 in this clinical setting. However, the data presented suggest that sST2 could be of use both as a diagnostic biomarker to establish the presence of asthma and a disease-monitoring biomarker to guide therapy or to detect disease exacerbations. These findings warrant further study given the strong need for useful biomarkers in this area.
Allergic rhinitis
The condition of allergic rhinitis often shares a common (atopic) genesis with asthma. Similarly, study of nasal mucosal polyps from patients with chronic rhinosinusitis has shown high expression of ST2. Kamekura et al also showed increased ST2 expression in mucosa of patients with allergic rhinitis, and concentrations of sST2 may be a biomarker for inflammation in allergic rhinitis in a similar fashion as asthma. In a study by Baumann et al, sST2 levels from nasal washings were significantly increased in pollen-exposed allergic volunteers, compared with control subjects (p = 0.002). Serum sST2 rose as well, although this was only tested in 6 subjects. Conversely however, Gluck et al found IL-33, but not ST2, to be elevated in sera of patients with allergic rhinitis, although patients were not tested specifically during exacerbation, and sST2 was not specifically measured.
Similar to asthma, these findings lend support to the important pathophysiological role of the ST2 pathway in this condition and suggest there may be a reasonable basis for further study to develop the role of sST2 as a clinical biomarker.
ST2 in Nonallergic Respiratory Disease
Interstitial lung disease
Tajima at al studied 49 patients admitted to a hospital for acute exacerbations of idiopathic pulmonary fibrosis and 200 healthy volunteers. They found that patients with stable disease had sST2 levels similar to those of the healthy controls, but that during exacerbations of fibrosis, sST2 rose significantly (p <0.001, acute exacerbation group, 2.76 ± 0.56 ng/ml; stable state group, 0.44 ± 0.07 ng/ml; healthy control group, 0.42 ± 0.03 ng/ml) and correlated directly with measurements of inflammation ( r = 0.344, p = 0.005 with lactate dehydrogenase and r = 0.496, p <0.001 with C-reactive protein). No other studies to our knowledge have explored the role of sST2 in fibrotic pulmonary disease.
Acute respiratory distress syndrome
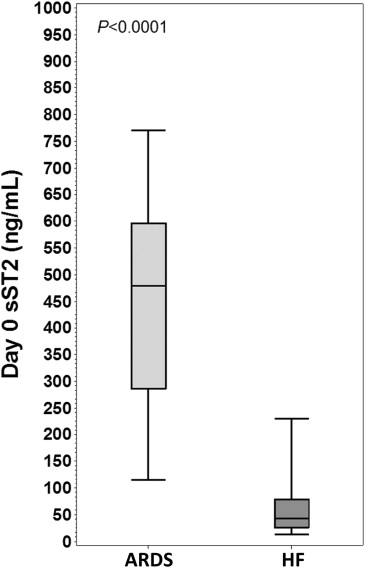
The authors of this report also conducted a large study to determine the characteristic of plasma sST2 concentrations (measured using the Presage ST2 assay) in 826 patients with the acute respiratory distress syndrome (ARDS), a condition that involves a profound inflammatory response in the lungs, in response to an injurious stimulus. These data showed that sST2 levels were markedly increased in patients with ARDS and that higher levels were associated with worse prognosis. Those dying from ARDS had greater day-0 (p <0.0001) and day-3 (p <0.0001) concentrations of sST2, and after adjustment for ARDS severity, sST2 concentrations predicted mortality at day 0 (adjusted odds ratio [OR] 1.47; 95% CI 0.99 to 2.20; p = 0.06) and day 3 (adjusted OR 2.94; 95% CI 2.00 to 4.33; p <0.0001), and if sST2 values rose from day 0 to day 3, an even greater risk was seen (adjusted OR 3.63; 95% CI 2.38 to 5.53; p <0.0001). Notably, cumulative fluid balance was more positive in patients with greater day-0 sST2 values (5,212 vs 2,020 ml, p <0.0001), with similar findings at day 3. Greater values for sST2 were independently associated with fewer ventilator-free days and intensive care unit–free days.
Interestingly, given the massive elevation of sST2 seen in ARDS (that often was substantially greater than that seen in patients with HF with alveolar edema), we hypothesized plasma sST2 might ultimately be helpful in the differential diagnosis of ARDS versus HF ( Figure 1 ). Further data in this regard are needed.