ST2 is a member of the interleukin (IL) 1 receptor family that exists in 2 forms, a transmembrane receptor (ST2L) and a soluble receptor (sST2). The ligand of ST2 is IL-33, known to be involved in reducing tissue fibrosis and myocyte hypertrophy in mechanically strained hearts. Through its ability to act as a decoy receptor, sST2 blocks the beneficial effects that occur when IL-33 attempts to bind to ST2L; experimentally, this leads to cardiac hypertrophy, fibrosis, and ventricular dysfunction. In patients with acutely decompensated heart failure, elevated concentrations of sST2 are strongly associated with the presence and severity of the diagnosis and powerfully predict increased risk of heart failure complications including arrhythmia, pump failure, or death, independent of natriuretic peptides and other established or emerging biomarkers. The role of sST2 measurement in acutely decompensated heart failure evaluation and management will be discussed.
The diagnosis of heart failure (HF) has achieved epidemic proportions in recent years; in the United States alone, it is presently estimated that 550,000 new HF cases develop per year, with an incidence expected to grow. The reasons for this global pandemic include an aging population, growing incidence of risk factors for ischemic heart disease, improved survival after myocardial infarction (MI), and reduction in other causes of premature death. Worsening the situation is that HF is highly morbid, accompanied by a high risk for mortality. Among the most pivotal moments in the arc of the course of HF is the development of acutely decompensated symptoms or signs of the diagnosis. In this setting, a substantial increase in the risk for recurrent HF events is present; such presentation marks patients at risk for repeat hospitalization, progressive myocardial remodeling, or death. Indeed, mortality rates after an HF hospitalization are 5% to 10% at 30 days and nearly 25% by 1 year.
Challenging the complexity of evaluation and management of the patient with acutely decompensated HF (ADHF) is that it is complex to estimate severity of the syndrome, with corresponding difficulties in prognostication; taken together, this may result in limitations in correct risk stratification and treatment, possibly resulting in worse outcomes for the patients affected by the diagnosis. Given the potential challenges posed by the patient with suspected or proven ADHF, clinicians have turned to tools to objectively supplement standard history and physical examination; in this regard, the use of biomarkers in patients with suspected or proven ADHF has grown over recent years.
The current gold standards for clinical HF biomarker testing are the natriuretic peptides (e.g., B-type natriuretic peptide, BNP and its amino-terminal cleavage fragment, NT-proBNP). Induced by pressure or volume overload, BNP and NT-proBNP are related to the severity of abnormalities in cardiac structure and function ; both BNP and NT-proBNP are now widely used to secure HF diagnosis and are supported for prognosis as well. Despite their value for evaluation and management of patients with ADHF, the natriuretic peptides are only a single biomarker in a complex pathophysiology that describes HF. In this regard, there has been considerable effort toward broadening the range of biomarkers for use in ADHF evaluation, with the idea of identifying markers reflecting complementary biologic information to that of BNP or NT-proBNP. Among the most promising is ST2.
ST2 as a Biomarker in Heart Failure
The biology of ST2 is reviewed elsewhere in the International ST2 Consensus (REF of biology section). However, briefly, ST2 is a member of the interleukin (IL) receptor superfamily and participates in a broad range of biologic processes relevant to cardiovascular disease. Originally identified as involved in regulating immunologic tolerance through induction of T-helper cell type 2 responses, ST2 is also known to be involved in the process of tissue fibrosis development through interactions with its ligand, IL-33, which may be bound by either a membrane-bound version of ST2 known as ST2 ligand (ST2L) or a soluble form known as soluble ST2 (sST2). Early data demonstrated that in the context of mechanical cardiomyocyte and cardiac fibroblast strain, ST2 and IL-33 gene transcription increases, with expression of ST2L and release of sST2 and IL-33. Binding of IL-33 to ST2L results in favorable antihypertrophic and antifibrotic effects in the myocardium; sST2 also binds IL-33 and is believed to represent a decoy receptor “off switch” by which the body regulates balance of the IL-33/ST2 pathway. However, in states of excessive sST2 secretion, benefit of IL-33/ST2L binding is lost, and progressive myocardial fibrosis and hypertrophy may occur, with substantial increase in risk for progressive HF and death in experimental models. Given the recognition that concentrations of sST2 could be measured in circulation of humans with HF along with the development and optimization of acceptable means by which to measure sST2 has led to numerous studies of its measurement in patients with HF.
ST2 Measurement in ADHF
The first study of sST2 measurement in patients with suspected or proven ADHF was in the Pro-BNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. In this analysis of 593 patients admitted to the emergency department with acute dyspnea (with and without HF), the investigators found concentrations of sST2 (measured with an early research-use-only assay) were significantly greater in patients with ADHF than patients with non-HF dyspnea (0.50 vs 0.15 ng/ml; p <0.001), and the likelihood for HF diagnosis was greater in patients in the highest deciles of sST2. However, NT-proBNP concentrations were significantly better than sST2 for diagnosis of ADHF.
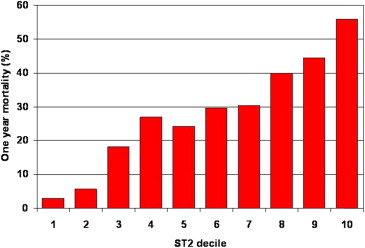
Notably, however, the prognostic meaning of sST2 was considerable in the PRIDE study: concentrations of the marker were greater in patients who were dead at 1 year than those in survivors (1.08 vs 0.18 ng/ml; p <0.001), and there was clear dose dependence between sST2 concentrations and risk of death at 1 year, with greater concentrations predicting substantial risk ( Figure 1 ). In multivariate regression analysis for predictors of death at 1 year, sST2 concentrations strongly predicted 1 year mortality in both patients with and without HF. Notably, the prognostic value of sST2 was additive to that of NT-proBNP, such that patients with elevations in both NT-proBNP and sST2 experienced the highest rate of mortality at 1 year, approaching 40%.
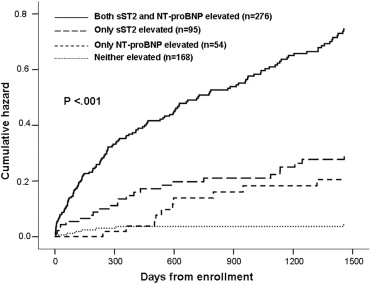
Examining patients as a function of sST2 and NT-proBNP values, the risk of death (regardless of HF vs non-HF cause of dyspnea) emerged almost immediately after enrollment and continued to increase over time. This association between sST2 and NT-proBNP with prognosis remains intact out to 4 years from presentation ( Figure 2 ).
In a subsequent study of 346 patients with acute HF from the PRIDE study and a similar cohort of patients from Linz, Austria, Rehman et al examined the association between sST2 concentrations and clinical characteristics and prognosis. sST2 concentrations at presentation correlated with New York Heart Association functional class, left ventricular (LV) ejection fraction ( r = 0.13), creatinine clearance ( r = 0.22), B-type natriuretic peptide ( r = 0.29), NT-proBNP ( r = 0.41), and C-reactive protein ( r = 0.43; all p <0.05). Unlike natriuretic peptides, sST2 levels were not related to age, previous diagnosis of HF, body mass index, atrial fibrillation, or cause of cardiomyopathy (ischemic vs nonischemic). As evidenced in the PRIDE study, sST2 levels were higher in patients with acute HF who died at 1 year; in multivariate Cox regression analysis of independent predictors of death, sST2 was associated with a 2-fold risk of mortality independent of other clinical and biochemical parameters of risk (including natriuretic peptide levels), and the impact of sST2 concentration on admission on 1 year mortality was similar in patients with preserved and depressed LV function. It is worthwhile to note that in this analysis, the presence of an sST2 value in the prognostic model led to NT-proBNP losing prognostic importance in those with HF with preserved ejection fraction (HFpEF).
Much as in the original PRIDE analysis, those with both an elevated sST2 and NT-proBNP level experienced the greatest risk of death at 1 year (>40%), whereas patients with both biomarkers below the median levels experienced a remarkably low mortality (<10%). Interestingly, a high sST2 level reclassified risk of death in patients with a low natriuretic peptide level, suggesting that sST2 significantly augments traditional markers of risk stratification in acute HF. Moreover, in patients with an ST2 value below the median concentration, NT-proBNP >1,000 pg/ml (a conventional risk threshold) did not forecast 1-year mortality.
ST2 Measurement in ADHF
The first study of sST2 measurement in patients with suspected or proven ADHF was in the Pro-BNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. In this analysis of 593 patients admitted to the emergency department with acute dyspnea (with and without HF), the investigators found concentrations of sST2 (measured with an early research-use-only assay) were significantly greater in patients with ADHF than patients with non-HF dyspnea (0.50 vs 0.15 ng/ml; p <0.001), and the likelihood for HF diagnosis was greater in patients in the highest deciles of sST2. However, NT-proBNP concentrations were significantly better than sST2 for diagnosis of ADHF.
Notably, however, the prognostic meaning of sST2 was considerable in the PRIDE study: concentrations of the marker were greater in patients who were dead at 1 year than those in survivors (1.08 vs 0.18 ng/ml; p <0.001), and there was clear dose dependence between sST2 concentrations and risk of death at 1 year, with greater concentrations predicting substantial risk ( Figure 1 ). In multivariate regression analysis for predictors of death at 1 year, sST2 concentrations strongly predicted 1 year mortality in both patients with and without HF. Notably, the prognostic value of sST2 was additive to that of NT-proBNP, such that patients with elevations in both NT-proBNP and sST2 experienced the highest rate of mortality at 1 year, approaching 40%.
Examining patients as a function of sST2 and NT-proBNP values, the risk of death (regardless of HF vs non-HF cause of dyspnea) emerged almost immediately after enrollment and continued to increase over time. This association between sST2 and NT-proBNP with prognosis remains intact out to 4 years from presentation ( Figure 2 ).
In a subsequent study of 346 patients with acute HF from the PRIDE study and a similar cohort of patients from Linz, Austria, Rehman et al examined the association between sST2 concentrations and clinical characteristics and prognosis. sST2 concentrations at presentation correlated with New York Heart Association functional class, left ventricular (LV) ejection fraction ( r = 0.13), creatinine clearance ( r = 0.22), B-type natriuretic peptide ( r = 0.29), NT-proBNP ( r = 0.41), and C-reactive protein ( r = 0.43; all p <0.05). Unlike natriuretic peptides, sST2 levels were not related to age, previous diagnosis of HF, body mass index, atrial fibrillation, or cause of cardiomyopathy (ischemic vs nonischemic). As evidenced in the PRIDE study, sST2 levels were higher in patients with acute HF who died at 1 year; in multivariate Cox regression analysis of independent predictors of death, sST2 was associated with a 2-fold risk of mortality independent of other clinical and biochemical parameters of risk (including natriuretic peptide levels), and the impact of sST2 concentration on admission on 1 year mortality was similar in patients with preserved and depressed LV function. It is worthwhile to note that in this analysis, the presence of an sST2 value in the prognostic model led to NT-proBNP losing prognostic importance in those with HF with preserved ejection fraction (HFpEF).
Much as in the original PRIDE analysis, those with both an elevated sST2 and NT-proBNP level experienced the greatest risk of death at 1 year (>40%), whereas patients with both biomarkers below the median levels experienced a remarkably low mortality (<10%). Interestingly, a high sST2 level reclassified risk of death in patients with a low natriuretic peptide level, suggesting that sST2 significantly augments traditional markers of risk stratification in acute HF. Moreover, in patients with an ST2 value below the median concentration, NT-proBNP >1,000 pg/ml (a conventional risk threshold) did not forecast 1-year mortality.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


