Biomarkers of cardiovascular diseases are indispensable tools for diagnosis and prognosis, and the use of several biomarkers is now considered the standard of care. New markers continue to be developed, but few prove to be substantially better than established markers. Suppression of tumorigenicity 2 (ST2) is a marker of cardiomyocyte stress and fibrosis that provides incremental value to natriuretic peptides for risk stratification of patients with a wide spectrum of cardiovascular diseases. On the basis of all available data, the 2013 American College of Cardiology and American Heart Association guidelines now recommend measurement of ST2 for additive risk stratification in patients with acute or chronic ambulatory heart failure (HF). This report provides an up-to-date overview of the clinical studies that led to the endorsement of ST2 as a cardiovascular prognostic marker in chronic HF. The presented data suggest that the addition of ST2 to a model that includes established mortality risk factors, including natriuretic peptides, substantially improves the risk stratification for death and HF hospitalization in patients with HF. ST2’s prognostic value remains strong even in the subset of patients with renal insufficiency and is superior to other remodeling-fibrosis biomarkers currently being evaluated. In conclusion, these results have been repeatedly validated; thus, ST2 could be rapidly incorporated into clinical practice for risk prediction. Indeed, the body of evidence supporting the use of ST2 in chronic HF stratification continues to grow, with consistent data from cohorts around the world in single-center (Barcelona, Brussels, and San Diego cohorts) and multicenter (Penn Heart Failure Study [PHFS] and Muerte Subita en Insuficiencia Cardiac [MUSIC]) studies and in post hoc studies from clinical trials (Prospective Randomized Amlodipine Survival Evaluation 2 [PRAISE-2], Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training [HF-ACTION], and Controlled Rosuvastatin Multinational Trial in Heart Failure [CORONA]).
Biomarkers have established roles in diagnosis and prognosis for a number of cardiovascular diseases. Natriuretic peptides in particular have evolved over the past 10 years to become part of the standard of care for evaluating patients with heart failure (HF) in acute and ambulatory settings. Despite the well-documented successes and strengths of natriuretic peptides, there is plenty of room for improvement in the evaluation and risk stratification of patients with HF or at risk for HF. Although many candidate biomarkers have been evaluated to help fill this gap, few have survived the rigorous studies that are a prerequisite to translation into the clinical realm. Suppression of tumorigenicity 2 (ST2) is a biomarker that has successfully navigated this course and is emerging as a bona fide tool for patient care. This report provides an up-to-date overview of the clinical studies that have led to the establishment of ST2 as a guideline-endorsed biomarker of cardiovascular risk.
The recent 2013 American Heart Association and American College of Cardiology guidelines for the management of HF provide a class IIb recommendation for the measurement of ST2 in patients with acute or ambulatory HF. The guidelines note that not only is ST2 predictive of hospitalization and death in patients with HF, but information gleaned from ST2 measures provides additive prognostic value to that from natriuretic peptides. In this article, we focus on the body of evidence regarding ST2 in chronic HF ( Table 1 ).
| First Author | Journal/year | Cohort | Population | Study Description | N | Mean Age (yrs) | Avg LVEF | Primary Outcomes | Follow-up Duration | Summary of Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Single-Center | ||||||||||
| Bayes-Genis | Eur J heart Fail 2012 | Barcelona | Chronic HF | ST2 and NT-proBNP | 891 | 70 | 34% | Death | 2.8 yrs | ST2 associated with mortality. NRI 9.9% |
| Daniels | Am heart J 2010 | SDVA Echo | Ambulatory pts referred for echo | ST2 at time of echocardiogram | 588 | 68 | 59% | Death | 1 year | ST2 associated with mortality |
| Gruson | Int J Cardiol 2014 | Brussels | ChronicHF | ST2, BNP, NT-proBNP and proBNP 1-108 | 137 | 67 | 23% | CV death | 4.2 yrs | ST2 predictive of long-term CV death |
| Multi-center | ||||||||||
| Ky | Circ heart Fail 2011 | PHFS | Chronic HF | ST2 + NT-proBNP on top of SHFM | 1141 | 56 | 32% | Death or cardiac transplant | 2.8 years | ST2 associated with mortality. NRI 14.9%. |
| Pascual-Figal | J Am Coll Cardiol 2009 | MUSIC | Chronic HF | ST2 + NTproBNP | 99 | 66 | 29 | Sudden cardiac death | 10.8 mo | ST2 associated with SCD |
| Post-hoc Clinical Trials | ||||||||||
| Weinberg | Circulation 2003 | PRAISE-2 | Chronic non-ischemic HF, NYHA class III-IV | ST2 and change in ST2 | 161 | 60 | 22% | Death or cardiac transplant | NA | 2 week change in ST2 associated with mortality and cardiac transplant |
| Felker | Circ heart Fail 2013 | HF-ACTION | Chronic HF | ST2 and functional capacity and clinical outcomes | 912 | 59 | <35% | Functiona capacity; death; hospitalization | 2.5 yrs | ST2 associated with worse outcomes |
| Broch | Eur J heart Fail 2012 | CORONA | Chronic ischemic HF | ST2, NT-proBNP, and CRP | 1449 | 72 | 32% | CV death, non- fatal MI, stroke | 2.6 yrs | ST2 associated with worsening HF and hospitalization |
Consistent with the data in acute HF, studies of patients with ambulatory HF have demonstrated the prognostic ability of ST2 across a wide range of cohorts ( Figure 1 ). In a multicenter study of 1141 patients with chronic HF, the Penn Heart Failure Study (PHFS) compared ST2 and N-terminal pro–brain natriuretic peptide (NT-proBNP) with the Seattle Heart Failure Model (SHFM) for the prediction of death or cardiac transplantation at 1 year. The combination of ST2 and NT-proBNP had risk discrimination similar to that of the SHFM. Although adding these biomarkers to the SHFM did not improve risk discrimination further, it significantly improved risk classification. In terms of assessing individual patient risk, ST2 alone performed as well as the established biomarker NT-proBNP but was not significantly better than the SHFM alone. Adding ST2 and NT-proBNP levels to the SHFM score improved risk discrimination by reclassifying 14.9% of patients into more appropriate categories.
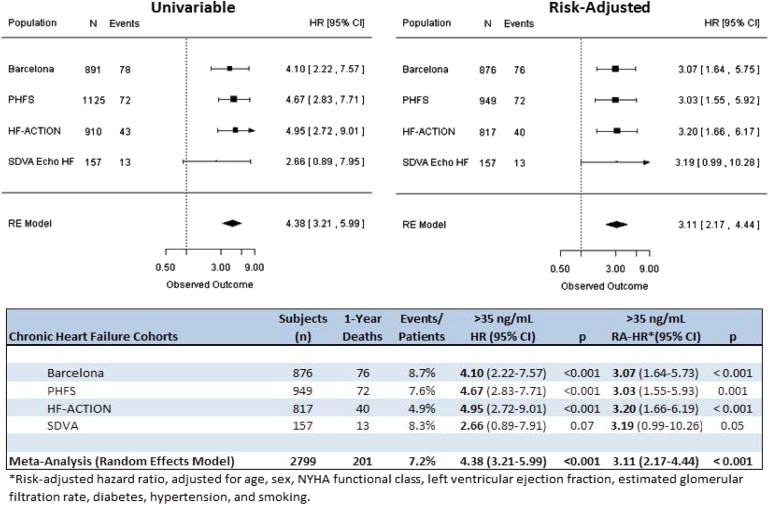
Taken together, these findings demonstrated that ST2 is a potent indicator of prognosis in chronic HF and offers a moderate improvement in risk stratification when used in combination with conventional markers. There was some evidence that ST2 levels were even more predictive of risk in patients with HF of nonischemic origin compared with those with ischemic HF.
The PHFS provided information that was complementary to the first assessment of ST2 in chronic HF, which was a substudy of Prospective Randomized Amlodipine Survival Evaluation 2 (PRAISE-2). The substudy reported on 161 patients with New York Heart Association (NYHA) class III or IV nonischemic HF and found that serial changes in ST2, but not baseline ST2 levels, were associated with increased risk for death or transplantation. In contrast, Ky et al found a robust, independent association between a single baseline measure of ST2 and the risk for adverse outcomes. The investigators argued that these differences are probably attributable to the use of a more sensitive ST2 assay, a larger sample size, and the assessment of ST2 in a broader spectrum of patients with HF.
ST2 and other HF biomarkers may have their greatest clinical utility in settings in which the components of the SHFM risk score, such as physician-assessed NYHA class and the left ventricular ejection fraction, are unavailable or in settings in which these data are available but the treating physician remains uncertain about the prognosis. Biomarkers may provide additional mechanistic insights into the biologic alterations that occur with HF, which is a complex syndrome.
The Barcelona ST2 Study used data from consecutive patients treated at a structured multidisciplinary HF unit to investigate whether a combination of ST2 and NT-proBNP improved the risk stratification of HF patients beyond an assessment based on established mortality risk factors, including age, gender, ischemic origin of HF, the left ventricular ejection fraction, NYHA functional class, diabetes, glomerular filtration rate, sodium, hemoglobin, and β-blocker and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker treatment. ST2 was measured with a novel high-sensitivity immunoassay. During a median follow-up time of 33.4 months, 244 of the 891 participants in the study died. In the multivariate Cox proportional-hazards model, ST2 and NT-proBNP significantly predicted the risk for death. The inclusion of ST2 and NT-proBNP separately in the model, along with established mortality risk factors, significantly improved the c -statistic for predicting death. The net improvement in reclassification after the separate addition of ST2 to the model with established risk factors and NT-proBNP was estimated to be a significant 9.90%.
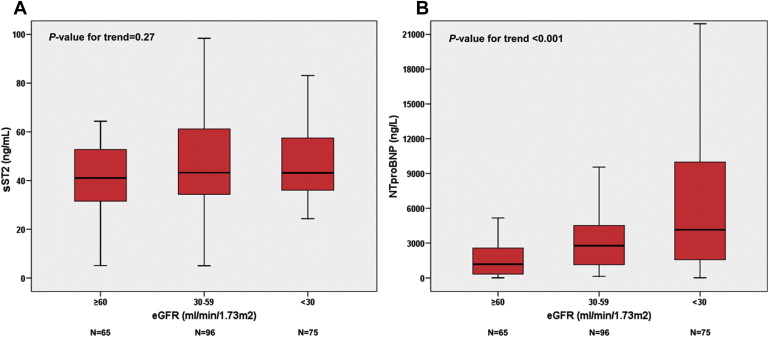
Importantly, in the Barcelona study, the prognostic value of ST2 was not influenced by renal function. The inclusion of ST2 with other biomarkers improved long-term prediction in patients with renal insufficiency even more than in the total cohort. Bayes-Genis et al found that in the total population, the ST2 serum concentration increased modestly as the estimated glomerular filtration rate (eGFR) worsened. However, in the sickest patients (NYHA functional classes III and IV), the ST2 serum concentrations were similar in the 3 eGFR strata (eGFR >60, 30 to 59, and <30 ml/min/1.73 m 2 ), while the NT-proBNP concentrations increased significantly with worsening eGFR ( Figure 2 ). Considering inflammatory status as secondary to HF and renal dysfunction, it is possible that ST2 was a stronger independent outcome predictor in this setting because ST2 reflects inflammation and cardiac strain. Considering these 2 findings (i.e., the weaker relation of ST2 serum concentrations with renal function and better stratification in long-term survival), the use of ST2 in patients with renal insufficiency might be preferable when only 1 of the biomarkers is available, especially in patients win NYHA functional classes III and IV.