Chapter 16 Spinal cord injury
INTRODUCTION
This view continued until the work of Guttmann and others encouraged development of special centres throughout the world and saw the problems associated with spinal cord injury at last being addressed, although mortality from tetraplegia until the 1960s remained at 35% (Grundy & Swain 1996). Improvements in administering care at the time of the accident, technological advances in diagnosis and management have contributed to a continuing fall in mortality and morbidity rates over recent years. Kemp & Krause (1999) state that there has been a 2000% increase in life expectancy following spinal cord injury in the past 50 years compared with a 30% increase for the non-disabled population. Mortality is significantly higher during the first year after injury, especially for those with higher-level injuries. Life expectancy tables and other facts and figures are available online from the National Spinal Cord Injury Statistical Center at the University of Alabama, USA (see Further reading).
In a 50-year study in the United Kingdom, Frankel et al (1998) found that 92.3% of spinal cord injuries survived, the primary cause of death being attributed to respiratory complications. Another study has shown a projected mean life expectancy of 84% of normal for paraplegia and 70% for tetraplegia (Yeo et al 1998).
The respiratory care of patients with spinal cord injury is discussed in this chapter. It should, however, be remembered that effective management of patients requires a holistic, multidisciplinary approach, preferably in a spinal cord injuries unit, to ensure optimal rehabilitation.
MECHANICS OF RESPIRATION AND THE EFFECT OF SPINAL CORD INJURY
Normal respiration
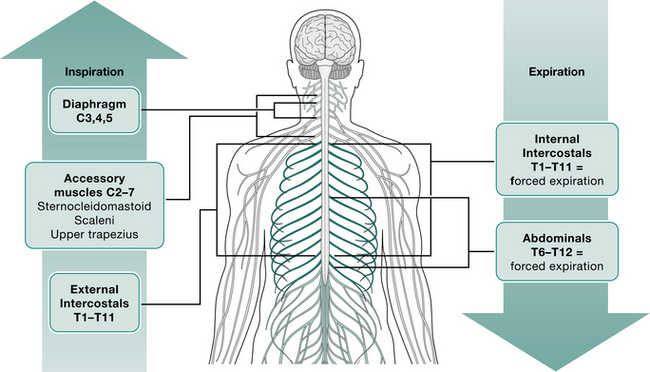
An understanding of normal respiratory mechanics is needed to appreciate the effect of a spinal cord injury. Figure 16.1 lists the muscles of respiration and their level of innervation.
The contraction and downward movement of the diaphragm and contraction of the intercostal muscles cause normal inspiration by generation of negative intrapleural and subsequent negative intrathoracic pressure (Lucke 1998). The intercostal muscles also work to stabilize the rib cage against the tendency for paradoxical inward movement caused by the negative intrathoracic pressure during inspiration.
Expiration is normally a passive process except during a forceful manoeuvre such as coughing or sneezing. This force is generated mainly by the abdominal muscles, assisted by the intercostals at large lung volumes (De Troyer & Heilporn 1980).
Respiratory complications are a major cause of death in the early stages of spinal injury (Lanig & Peterson 2000, Van Buren et al 1994), and those at the highest risk are:
Effects of spinal cord injury
Following a spinal cord injury the muscles below and, not uncommonly, at the level of the injury are paralysed. The higher the level of injury, the greater the effect on respiration (Linn et al 2000). To gain a more precise picture of the muscles affected, the physiotherapist should refer to the level of innervation of the respiratory muscles and relate this to the neurological level of injury.
Tetraplegic patients (i.e. those with an injury affecting T1 or above) will have lost the use of their intercostal muscles, which has a profound effect on respiratory function. Depending on the level of their injury, only part of the diaphragm may be spared, yet it has to provide them with all of their respiratory effort (Cohn 1993).
Paradoxical breathing
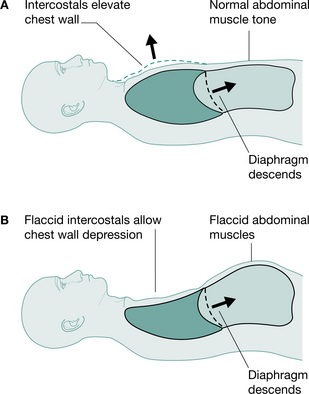
Tetraplegic patients injured at or below C4 will have partial or total innervation of the diaphragm and some accessory muscles of respiration and can be totally independent of mechanical ventilation. However, in the initial spinal shock stage, tetraplegic patients will have paralysis of their intercostal muscles, which, when flaccid, causes disruption to the mechanics of respiration. The usual splinting function of the intercostal muscles is lost and the negative intrathoracic pressure during inspiration causes paradoxical inward depression of the ribs (Lucke 1998, Menter et al 1997) (Fig. 16.2). This may lead to microatelectasis and an increase in the work of breathing (Fishburn et al 1990). With time the tendons, ligaments and joints of the rib cage stiffen owing to decreased active movement. This, together with spasticity of the intercostals, will provide some compensation for the loss of active control of these muscles and stabilize the rib cage, so that paradoxical breathing lessens (Axen et al 1985, Mansel & Norman 1990).
Cough
The ability to produce an effective cough is severely impaired in patients with cervical or high thoracic spinal cord injury (Roth et al 1997, Wang et al 1997). This is most marked when the intercostals are flaccid and the rib cage is at its most mobile. Patients who have loss of innervation to the abdominal muscles and the internal intercostals lose the ability to produce a forced expiration (Gouden 1997). De Troyer & Estenne (1991) have shown that patients with injuries at C5-8 can utilize the clavicular portion of pectoralis major to generate an expulsive force, although the extent to which this is functional is not clear. Linn et al (2000) found that in a group of patients with high tetraplegia (above C5) loss of peak expiratory flow rate was greater than 50% predicted. An effective cough for these patients requires external compression to produce the necessary large intrathoracic pressures; assisted coughing is discussed later.
The effect of position
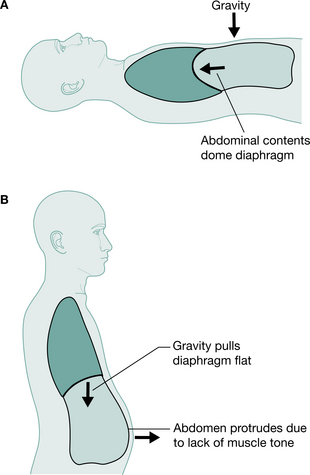
In the normal subject, mechanisms exist to ensure that adequate ventilation is maintained in all positions. In the supine position, contraction of the diaphragm displaces the abdominal contents without significantly expanding the rib cage, as the abdomen is more compliant than the rib cage. In standing, abdominal tone increases to support the abdominal contents, thereby decreasing abdominal wall compliance. Contraction of the diaphragm, intercostal and accessory muscles causes greater rib cage expansion, resulting in an increase in vital capacity in standing of about 5% (Chen et al 1990).
Positional changes will, however, affect the respiratory function of the tetraplegic patient. In supine, the weight of the abdominal contents forces the diaphragm to a higher resting level so that contraction produces greater excursion of the diaphragm. In sitting or standing, the weight of the unsupported abdominal contents increases the demand on the diaphragm, which now rests in a lower and flatter position (Chen et al 1990, Lucke 1998), decreasing effectiveness and restricting available excursion for creating negative intrapleural pressure (Fig. 16.3). Chen et al (1990) recorded a 14% drop in predicted vital capacity in the tetraplegic patient on changing position from supine to sitting or standing. Conversely, vital capacity of a tetraplegic patient rises by 6% when the bed is tipped 15° head down from supine (Bromley 1998). Linn et al (2000) showed a statistically significant decrease in forced vital capacity (FVC) in the erect position as compared with supine. It is therefore important with these patients not to assume that their respiratory ability will be sufficient in all positions.
Abdominal binders
Elasticated abdominal binders have been used on patients with high spinal cord injury for many years, both to minimize the effect of postural hypotension and aid respiration (Goldman et al 1986, McCool et al 1986, Scott et al 1993). Their effect is achieved by providing support to the abdominal contents, decreasing the compliance of the abdominal wall and thereby allowing the diaphragm to assume a more normal resting position in the upright posture (Alvarez et al 1981). Goldman et al (1986) investigated the effect of abdominal binders on breathing in tetraplegic patients and concluded that in the supine position there was no change, but when sitting there was a trend for improvement in lung volumes. This may help the patient considerably during the early stages of mobilization.
Sleep-disordered breathing and sleep apnoea syndrome after spinal cord injury
Sleep apnoea has been widely reported in patients with spinal cord injury and is known to be twice as prevalent as compared with the general population (Cahan et al 1993, McEvoy et al 1995, Young et al 1993). Stockhammer and colleagues (2002) defined two factors of sleep-disordered breathing and sleep apnoea. If a patient had more than 5 apnoeas per hour of sleep – the apnoea index (AI) and a respiratory disturbance index (RDI) of 15 or more, they were defined as having sleep apnoea syndrome (SAS). If their RDI was 15 or more but AI was less than 5 then they were defined as having sleep disordered breathing (SDB). In this study of tetraplegic patients 62% had SDB. Forty-eight per cent (55% of the men studied and 20% the women) had SAS. This is comparable with other studies that have found the prevalence of SAS to be 40% (Burns 2000) and 45% (Short et al 1992). Stockhammer et al (2002) found no correlation of level of lesion or spirometry values with the presence of RDI. They did, however, find a significant correlation of age, time since injury, body mass index (BMI) and neck circumference with RDI. They concluded that SAS incidence is high in tetraplegia, especially in older men with long-standing spinal cord injury. They also noted that patients with a high RDI did not complain of daytime drowsiness and were not obese, and therefore these individuals may not be investigated for SAS. Graham et al (2004) reported on two paraplegic men who had sleep apnoea who were aged 54 and 55 and were 37 and 25 years, respectively, post injury.
Berlowitz et al (2005) studied patients during the first year after cervical spinal cord injury. They found a high prevalence of SDB, which was first apparent 2 weeks following injury (60%) and peaked at 13 weeks or 3 months (83%). This then improved but remained significantly elevated (62%) at 1-year post injury. It should be noted that these outcomes cannot be compared directly with the studies quoted above, because this study reported on SDB whereas the others reported on SAS. Treatment for SAS with non-invasive ventilation is discussed later.
RESPIRATORY ASSESSMENT
Accurate assessment and regular review of respiratory status are vital. Initial assessment must be carried out as soon as possible to establish a baseline against which future deterioration or improvement can be monitored. Assessment is discussed in Chapter 1; however, in patients with spinal cord injury, the following details should also be considered:
PHYSIOTHERAPY TREATMENT
Respiratory management of the patient with spinal cord injury requires the application of the same principles as other respiratory problems; the skills used are discussed in Chapters 4 and 5. The goals of treatment include:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree