Fig. 5.1
Structures of SP-A and SP-D. Monomeric and oligomeric structures of SP-A and SP-D. Monomeric structures can be conceptually divided into four major structural domains: a short N-terminal segment containing two intermolecular disulfide bonds, a collagen-like sequence of Gly-X-Y repeats, an acidic and hydrophobic neck domain, and a C-terminal carbohydrate recognition domain (CRD). The CRD contains a calcium-dependent, carbohydrate-binding site. The matured molecule of SP-A and SP-D contains six and four trimeric subunits, respectively
5.2.2 Biochemical and Biological Functions of SP-A and SP-D
Within the lung, SP-A and SP-D are produced in alveolar type II cells and Clara cells. These pulmonary collectins bind to mannose, maltose, glucose, and fucose with higher affinities [24, 25]. SP-A interacts with surfactant phospholipids including dipalmitoylphosphatidylcholine (DPPC) and glycosphingolipids, such as galactosylceramide, in addition to the carbohydrates [26, 27]. SP-D also binds to phosphatidylinositol (PI) and glucosylceramide [28, 29].
Pulmonary collectins have been shown to function as host defense lectins [30]. The proteins have been shown to bind gram-negative bacteria including Escherichia coli, Klebsiella pneumoniae, and Haemophilus influenzae, gram-positive organisms such as Streptococcus pneumoniae, fungi including Aspergillus and Candida, and other pathogens including Mycobacterium tuberculosis, Pneumocystis carinii, and influenza virus. SP-A is thought to bind to pathogens via its CRD [31, 32]. The in vivo studies clearly reveal that pulmonary collectins are involved in bacterial clearance. SP-A binds to and enhances the phagocytosis of bacteria including Staphylococcus aureus, Klebsiella pneumoniae, Mycobacterium tuberculosis, and Haemophilus influenzae by alveolar macrophages [33]. SP-D also binds to microorganisms and aids opsonization and phagocytosis by alveolar macrophages [34]. Pulmonary collectins have been shown to function as inflammatory modulators in the lung. Pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS) and peptidoglycan, are potent stimulators of inflammatory cytokine secretion. Pattern recognition receptors, including Toll-like receptor (TLR) and CD14, are responsible for the recognition and signaling of PAMPs and cytokine production. A previous study demonstrated the different actions of SP-A for distinct serotypes of LPS. SP-A inhibits the macrophage-derived TNF-α secretion induced by smooth LPS, which is a complete structural form of LPS. On the other hand, SP-A does not attenuate or even augment TNF-α secretion elicited by rough LPS, which does not express O-specific antigen and is an SP-A ligand. These distinct effects of SP-A occur through the direct interaction of SP-A with CD14 [35]. SP-A also inhibits TNF-α secretion by gram-positive bacteria via the interaction with SP-A and TLR2, which recognizes peptidoglycan, a major cell wall component of gram-positive bacteria [36]. Taken together, lung collectins exhibit both inflammatory and anti-inflammatory functions. These distinct activities depend on the ligand-binding specificities of collectins.
5.3 What Molecule Is KL-6?
Anti-KL-6 monoclonal antibody (mAb) was first developed to recognize sialylated sugar chains as a serum tumor biomarker for pulmonary, breast, and pancreatic cancers. Although the precise epitope structure recognized by the anti-KL-6 mAb was unclear, the possible carbohydrate epitopes have been reported to be novel O-linked glycans containing 6′sulfo-Gal/GalNAc of MUC1 [37]. Hirasawa et al. reported that KL-6 was a submolecule of MUC1 based on the results of a carbohydrate composition analysis [38]. KL-6/MUC1 is commonly used to denote the KL-6 molecule.
MUC1 is a large glycoprotein containing three domains: (1) a cytoplasmic tail, (2) a single transmembrane region, and (3) an extracellular domain. The extracellular region of MUC1 contains sites of O- and N-linked glycosylation and variable number tandem repeat (VNTR) domains with 20–100 repeats of a 20-amino-acid sequence. MUC1 has an extended, rigid structure protruding 200–500 nm above the plasma membrane and is found on the apical surface of normal glandular epithelial cells (Fig. 5.2) [39, 40].
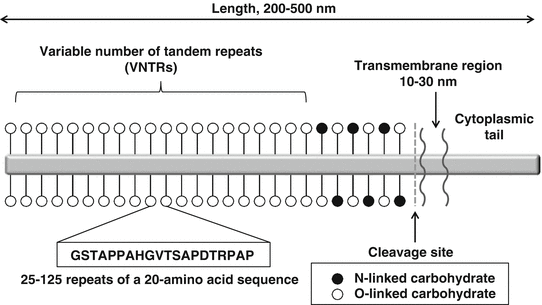

Fig. 5.2
Structure of MUC1. MUC1 is a large glycoprotein that contains three major structures: a cytoplasmic tail, a single transmembrane region, and an extracellular domain. The extracellular region contains sites of O- and N-linked glycosylation and variable number tandem repeat (VNTR) domains of 20–100 repeats of a 20-amino-acid sequence
5.3.1 Biochemical and Biological Functions of KL-6
KL-6 is normally expressed on the apical surface of glandular epithelial cells in many types of tissue including the breast, lung, and ovary. In the normal lung tissue, it is produced in alveolar type II cells and respiratory bronchiolar epithelial cells and is weakly expressed in basal cells of the terminal bronchial epithelium [41, 42]. KL-6 can be cut and released from the cell surface through the action of TNF-α converting enzyme (TACE; also called a disintegrin and metalloproteinase 17 [ADAM17]) and potentially ADAM9 [40, 43]. In addition, some soluble KL-6 may result from alternative splicing. In a transfection study using breast cancer cells, MUC1 prevented E-cadherin-mediated cell-cell and cell surface adhesion [44]. Another study demonstrated that anti KL-6 mAb mediates capping of MUC1 and restores E-cadherin, leading to inhibition of tumor proliferation [45]. These results suggested that KL-6 may be a target molecule of cancer therapy. In relation to the pathological role of lung fibrosis, previous reports have shown that KL-6 is one of the chemotactic factors for fibroblasts and has both proliferative and antiapoptotic effects for fibroblasts [38, 46]. These results indicate that KL-6 may stimulate fibrotic processes in interstitial lung diseases and support the theory that KL-6 is one of the key molecules involved in the intra-alveolar fibrotic process of pulmonary fibrosis.
5.4 How Do SP-A, SP-D, and KL-6 Appear in the Bloodstream?
Although surfactant proteins had been believed to be solely in the lungs, Chida et al. reported that surfactant proteins could be found in the sera of patients with RDS using a competitive ELISA with polyclonal antibody against SP-A or SP-B. A positive result for SP-A was obtained in four infants with RDS at 1 week of age and one surfactant-treated infant. All sera obtained at 2 months of age were negative for SP-A and SP-B. The specificities of antibodies and the presence of surfactant proteins in serum were not demonstrated in this study [47]. The enzyme-linked immunosorbent assay (ELISA) with two mAbs (PC6 and PE10) to human SP-A has been applied to the sera of patients with interstitial lung diseases. The sandwich ELISA is capable of determining an SP-A level ranging from 2 to 250 ng/mL when native SP-A isolated from patients with pulmonary alveolar proteinosis is used as a standard [15, 48]. Since human SP-A has been found to contain group A antigenic determinants, the criticism may be raised that monoclonal antibodies PC6 and PE10 may recognize simply group A antigen but not SP-A in the blood samples. When human serum is applied to an affinity column on mannose-sepharose and the serum proteins binding to the mannose-affinity matrix are analyzed by immunoblotting using anti-SP-A monoclonal antibody or anti-SP-D monoclonal antibody, the fraction with lectin activity contains approximately 35 kDa protein and 43 kDa protein, which correspond to the molecular sizes of SP-A and SP-D, respectively, purified from bronchoalveolar lavage fluid (BALF) [49]. This demonstrates that SP-A and SP-D exist in the bloodstream. One study has experimentally demonstrated that SP-A leaks from alveolar spaces into vessels. Human recombinant SP-A and/or artificial surfactant was intratracheally injected into immature newborn rabbits, and human SP-A in alveolar washings and sera was monitored by ELISA with PC6 and PE10, which do not cross-react with rabbit SP-A. The group that intratracheally received human SP-A and saline showed that 2.4 % of the human SP-A which was instilled into the lungs was detected in sera by ELISA. Since the group receiving saline alone showed no detectable human SP-A in sera, this study clearly demonstrates that SP-A leaks from alveolar space into the bloodstream [50]. Although the exact mechanism for the increase of SP-A and SP-D in sera of patients with interstitial pneumonia remains unknown, it is probably a combination of a loss of epithelial integrity due to injury and an increased mass of alveolar type II cells due to hyperplasia (Fig. 5.3).
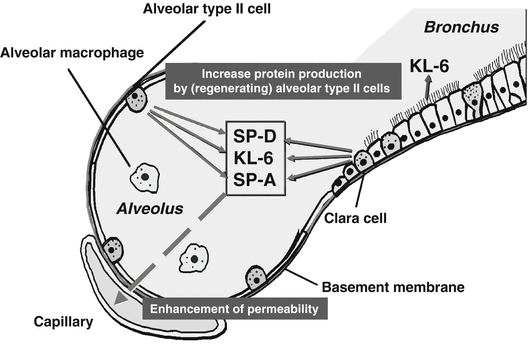

Fig. 5.3
Mechanism for the blood uptake of SP-A, SP-D, and KL-6. The increased serum levels of SP-A, SP-D, and KL-6 may be due to an increase in production of these proteins by (regenerating) alveolar type II cells and enhanced permeability following the destruction of the alveolar-capillary barrier
Regenerating alveolar type II cells are the main cellular source of KL-6 in the lungs of patients with interstitial pneumonias, including IPF and KL-6, which are present at high levels in BALF [51]. A correlation between KL-6 levels measured in BALF and in serum was shown in patients with interstitial pneumonia. In patients with chronic beryllium disease, KL-6 serum levels correlated with albumin levels in BALF [52]. These results demonstrated that KL-6, which was produced in the lungs, appeared in the bloodstream as well as lung collectins and that serum KL-6 levels reflected the permeability of the air-blood barrier (Fig. 5.3). Since KL-6 is an extremely high molecular weight glycoprotein, both the destruction of the alveolar-capillary barrier and the enhancement of alveolar-capillary permeability are thought to be necessary for the leakage of KL-6 into the bloodstream.
5.5 Utility as Biomarkers for Screening and Monitoring of Patients with IPF
The mean level of SP-A in sera from 323 healthy control subjects was estimated to be 24.6 ± 9.6 ng/mL [53], and there was no difference in the levels stratified by gender and age [54]. However, the SP-A levels tend to be slightly higher in cigarette smokers [55]. The mean level of SP-D in sera from 129 healthy control subjects was 49 ± 24 ng/mL, and there was also no difference between SP-D levels by gender and age [56]. We reported that the serum SP-A and SP-D levels in patients with IPF were increased: mean levels of 77.6 ± 47.6 ng/mL and 303 ± 220 ng/mL (n = 57), respectively. When the cutoff values (mean ± 2SD of healthy control subjects) were set at 43.8 ng/mL for serum SP-A and 109.8 ng/mL for serum SP-D, IPF patients showed high sensitivities for SP-A (78 %) and SP-D (87 %). These values were extremely high in comparison with lactate dehydrogenase (LDH) (17 %), which is not specific to the lungs and is released from many organs [13–15, 48, 56].
Kohno et al. reported that the average serum levels of KL-6 for 160 healthy control subjects was 258 ± 131 U/mL (mean ± SD). When the upper limit of normal range was set at 520 U/mL (mean ± 2SD), the positive late for idiopathic interstitial pneumonia, including IPF, is 74 % (28 of 38) [42].
It should be noted that serum levels of these three markers increase in some diseases and are not specific to IPF. Previous studies have demonstrated that the levels of SP-A, SP-D, and KL-6 are elevated in sera from patients with IPF and other interstitial lung diseases (ILDs) such as collagen vascular disease (CVD)-ILD, radiation-induced pneumonitis, pulmonary alveolar proteinosis, and ARDS [14, 15, 48, 57–60]. Thus, these biomarkers may reflect alveolar epithelium cell dysfunction in a broad sense, which may not be specific to the pathogenesis of IPF. In addition, patients with advanced stages of lung cancer show high concentrations of these biomarkers in sera. Serum KL-6 is also elevated in patients with advanced stages of pancreatic and breast cancers [41, 61]. Serum SP-A, SP-D, and KL-6 are also elevated in some infectious diseases such as pneumocystis and cytomegalovirus pneumonia [62, 63].
With regard to monitoring of clinical course, acute exacerbation is an extremely important phenomenon. We reported that the most common cause of death in Japanese IPF patients was acute exacerbation with a frequency of 40 % in a large-scale epidemiological survey [64]. It is important to promptly know about the occurrence of acute exacerbation and to start optimal therapy. High-resolution computed tomography (HRCT) is a reliable examination method for detecting acute exacerbations; however, it is not always possible to be repeated at frequent intervals. The serum levels of these biomarkers increase without exception when patients with IPF develop respiratory failure due to acute exacerbation [13, 65]. In contrast, IPF patients developing respiratory failure due to bacterial infection seldom show high levels of these biomarkers. Therefore, the measurements of these biomarkers may support the differential diagnosis for causes of acute respiratory failure. Moreover, in many patients, the levels concomitantly decline promptly with clinical improvement, suggesting that these biomarkers may be reliable monitoring markers.
5.6 Utility as Biomarkers for Evaluating Disease Activity and Predicting Prognosis of Patients with IPF
It is an important role for biomarkers to predict prognosis because patients with IPF have considerable individual variability in clinical course of disease. Our study demonstrated that high levels of serum SP-A and SP-D were associated with mortality in patients with IPF. Fifty-two IPF patients were studied to evaluate the association between serum SP-A and SP-D and deterioration in pulmonary function and survival during 3 years of follow-up. The SP-D concentration, unlike that of SP-A, was related to the rate of deterioration per year in pulmonary function. The concentrations of SP-A and SP-D in patients who died within 3 years were significantly higher than in patients who were still alive after 3 years. Initial SP-A levels in non-survivors (117.7 ± 66.8 ng/mL) were significantly higher than those in survivors (68.8 ± 40.4 ng/L) (p = 0.0125). Initial SP-D levels in non-survivors (453.7 ± 290.3 ng/mL) were also significantly higher than those in survivors (248.0 ± 176.4 ng/mL) (p = 0.0032) [13]. In a study using Cox’s proportional hazards model, Greene et al. found that the levels of serum SP-A (log of SP-A, HR, 1.73; p = 0.031) and SP-D (log of SP-D, HR, 2.04; p = 0.003) in patients with IPF (n = 142) were significant predictors of mortality after adjusting for smoking history and age [14]. These studies were performed before ATS/ERS consensus classification for the diagnosis of IPF and might have included patients with nonspecific interstitial pneumonia [2]. Kinder et al. found that serum SP-A levels (each increase of 48.7 ng/mL, HR, 3.27; p = 0.003) were associated with an increased risk of 1-year mortality after controlling for known clinical predictors. There was no significant association between SP-D level and mortality, although addition of SP-D to SP-A improved 1-year mortality prediction significantly (area under the curve, 0.89 vs 0.76; p = 0.03) [66].
Satoh et al. reported that patients with higher levels of KL-6 had increased risk of mortality in patients with ILD [67]. During the study period (1999–2005), 219 patients (152 patients with IIP and 67 patients with CVD- ILD) were enrolled in this study. On HRCT scan, 183 patients showed an IPF/usual IP pattern. The median follow-up period of the 219 patients was 20 months. Overall mortality was 26.5 %. Serum KL-6 levels in the 58 non-survivors (median, 1330 U/mL) were significantly higher than those of the 161 survivors (median, 823/U/mL) (p = 0.0004). On the basis of receiver operating characteristic (ROC) analysis, the optimal point on the ROC curves for discriminating between survivors and non-survivors corresponding to KL-6 was 1000 U/mL. When the optimal cutoff level of 1000 U/mL was applied, its sensitivity was 67.2 % and specificity was 60.2 %. Yokoyama et al. reported a correlation between serum KL-6 levels and survival [16]. Twenty-seven patients with IPF were assessed retrospectively. During the 3 years of observation, 10 of 27 patients died. On the basis of univariate logistic correlation analysis, both KL-6 and LDH had significant correlations with survival among the six variables (age, VC% predicted, PaO2, C-reactive protein, LDH, and KL-6). Furthermore, multivariate analysis revealed that KL-6 but not LDH predicted prognosis. When the optimal cutoff level of 1000 U/mL was applied, survival was significantly different between the two groups (median survival, 36 months vs 18 months).
Song et al. hypothesized that a combination of biomarkers may be a more accurate predictor than any single biomarker alone [17]. In 118 patients with IPF, the predictive power of serum levels of biomarkers (SP-A, SP-D, KL-6, and MMP-7) and the predictive power of biomarkers in combination for clinical outcomes were shown. The data showed that blood levels of MMP-7 and SP-A are useful predictors of mortality and disease progression in IPF. The combination of both biomarkers yielded only marginally better prediction than clinical parameters alone; however, with addition of three biomarkers (MMP-7, SP-A, and KL-6), the improvement in predictability became statistically significant [17].
5.7 Relation Between HRCT Findings and Biomarkers
HRCT scanning is widely recognized to be a gold standard in determining disease activity and extent of pulmonary fibrosis. The HRCT pattern of IPF commonly shows patchy, predominantly peripheral, subpleural, bibasal reticular abnormalities. There may be a variable amount of ground-glass opacity (GGO) and alveolar opacity (AO). In areas of more severe involvement, there is often reticular opacity, traction bronchiectasis (TBE), and subpleural honeycombing (HCMB). The GGO observed on HRCT in patients with IPF can be associated with alveolar inflammation, mild fibrotic thickening of alveolar septa, and intraluminal fibroblastic foci. Areas of GGO often progress to reticular opacity or HCMB on follow-up evaluation.
We evaluated HRCT findings from 49 IPF patients to assess the correlation between scoring of HRCT findings and serum SP-A and SP-D levels [13]. In this study, the extent of GGO correlated significantly with serum levels of SP-A and SP-D (SP-A, ρ = 0.791, p < 0.0001; SP-D, ρ = 0.446, p < 0.0001, p = 0.0034, when analyzed using Spearman’s rank correlation test), whereas the extent of HMCB did not correlate with levels of either surfactant proteins. Next, we divided the IPF subjects into two subgroups: GGO-dominant type and parenchymal collapse opacity (PCO), which was defined as air bronchiolograms with intense lung attenuation with parenchymal collapse, often accompanied by thickened vessels and traction bronchiectasis. PCO may reflect collapsed and fibrotic abnormalities in peripheral airspaces of alveoli and bronchioles. SP-A levels (51.3 ± 33.3 ng/mL) in PCO-dominant type were significantly (p = 0.0003) lower than those (98.3 ± 55.8 ng/mL) in the GGO-dominant type, whereas SP-D levels were not significantly different for two types (GGO-dominant type, 243.1 ± 142.4 ng/mL; PCO-dominant type, 266.6 ± 161.1 ng/mL). The sensitivity of SP-A (52 %) was inferior to SP-D (83 %) in the PCO-dominant group. This may explain why SP-D is superior to SP-A in detecting mild interstitial changes, and mechanisms of increases in these proteins may differ.
5.8 Mechanism and Significance for Dissociation Among Serum Biomarker Levels
Simultaneous measurement of the serum levels of SP-A, SP-D, and KL-6 in patients with IPF sometimes reveals a dissociation among these serum biomarkers. For instance, an increase in the serum levels of SP-A and SP-D even in early and mild lung injury is often observed, while serum KL-6 levels remain unchanged [68]. The increases in serum KL-6 mean a leakage of high molecular weight protein from the alveolar space into the bloodstream, and that indicates an intense destruction of the alveolar-capillary barrier. It is believed that the discrepancy between serum KL-6 and lung collectins reflects the difference in the extent of alveolar epithelium damage. As another mechanism for dissociation, the dissociation between KL-6 and collectins in patients with acute eosinophilic pneumonia (AEP) may be a good case. Serum SP-A and SP-D are present at quite high levels; however, serum KL-6 remains at low level in AEP patients [69]. Lung collectins are secretory proteins, while KL-6 is basically a structural component of the cell membrane and its extracellular domain binds to the cell surface. Therefore, the presence of some proteinase, such as ADAM17 or ADAM9, to cleave its extracellular domain, may be essential for liberation into the alveolar space. If enzyme activity is lacking, KL-6 will be limited on the cell surface even though severe interstitial damage exists. BALFs from IPF patients show high concentrations of KL-6; however, KL-6 in the BALFs from AEP patients are within normal range, suggesting the mechanism responsible for the difference may be associated with the secretion manner of these proteins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


