and Iris Parrini6
(5)
Cardiology Unit, Service Department, IRCCS Candiolo – Turin, Turin, Italy
(6)
Cardiology Department, Mauriziano Hospital, Turin, Italy
Acute cardiotoxicity is an event occurring during the first administration of a chemotherapy agent or immediately after. It has to be differentiated from a hypersensitivity reaction. The most common expressions of acute toxicity are acute heart failure, acute coronary syndrome, arrhythmias, and hypertension. Symptoms, signs, and clinic expressions are according to the kind of toxicity.
The definition of cardiotoxicity is that of damage that affects the heart due to a toxic effect of a toxic substance or a drug.
Acute cardiotoxicity is usually defined as a cardiac event that arises during the first administration of a chemotherapy agent or immediately after.
This event is different from hypersensitivity reactions: in an observational study [1] during a 3-year period, a total of 240 infusion acute hypersensitivity reactions were collected out of 56,120 administrations performed, with an overall incidence of 0.4 %. In order of frequency, platinum derivatives, taxanes, and monoclonal antibodies accounted for the highest incidences. Their relative frequency was oxaliplatin, 2.5 %; carboplatin, 0.4 %; paclitaxel, 1.2 %; docetaxel, 1.2 %; trastuzumab, 1.2 %; and rituximab, 1.2 %.
The most studied acute cardiovascular reaction is described with anthracyclines.
Fortunately, acute cardiotoxicity is very rare: it occurs in <1 % of patients immediately after infusion of anthracycline therapy, and it manifests as an acute, transient decline in myocardial contractility, which is usually reversible [2]. If it is of little entity, it cannot be identified by the patients due to its very light discomfort. If we consider toxicity within 1 week from administration, the incidence rises to 11 % [9].
Some chemotherapy agents other than anthracyclines may have acute cardiac toxic effects.
The drugs that can cause acute cardiotoxicity are summarized in Table 6.1 (from De Vita et al.) [3].
Table 6.1
Drugs that can cause acute cardiotoxicity: onset, incidence, and CV effects
Drug | Onset | Incidence (%) | CV effects |
|---|---|---|---|
Amsacrine1 | Rare | H, AR, VR, CHF | Acute, subacute |
Arsenic trioxide | 3–24 | QT, VR | Acute |
Bevacizumab | 8–67 | HBP rare, CHF | Acute, subacute |
Bortezomib | 2–5 | CHF, H, QT | Acute, subacute |
Busulfan | Rare | CHF | Late |
Carmustine | Rare | H, AR, CP | Acute |
Cisplatin | Rare | H, AR, VR, CP | Acute |
Clofarabine | 27 | CHF, HBP, H | Acute, subacute |
Cyclophosphamide | <10 | CHF | Acute |
Cytarabine | Rare | AR, VR, CP, P, CHF | Acute, subacute |
Dasatinib | <1–3 | QT, CHF, HBP | Acute |
Erlotinib | 2.3 | CP, MI | |
Etoposide | 1–2 | H | Acute |
Rare | CP, MI | ||
Gemcitabine | Rare | AR, CHF | Acute |
Interferon | Rare | H, AR, CP, MI, CHF | Acute, subacute |
Interleukin-2 | Dose dependent | H, AR, CP, MI | Acute, subacute |
Lapatinib | Rare-16 | QT | |
Mechlorethamine | Rare | AR | Subacute |
Mitomycin | 10 | CHF | |
Nilotinib | 1–10 | QT | |
Paclitaxel | 0.5 | AR, VR | Acute |
Pentostatin | 3–10 | AR, VR, CP, MI, CHF | Subacute |
Sorafenib | 17–43 | HBP | Acute, subacute |
2.7–3 | CP, MI | ||
Sunitinib | 5–47 | HBP | Acute, subacute |
2.7–11 | CHF, MI | ||
Teniposide | 2 | H | Acute |
Tretinoin | 14–23 | H, AR | Acute, subacute |
3–6 | CP, MI, P, CHF | Acute, subacute | |
Vandetanib | Rare | CHF | Acute |
Vinca alkaloids | 10 | H | Acute, subacute |
Rare | MI | ||
Vorinostat | 3.5–6 | QT |
The discontinuation of the therapy usually is followed by improvement of cardiac function; sometimes cardiac damage may persist despite withdrawal of therapy.
6.1.1 Symptoms
Dyspnea: usually is present at rest with or without oxygen desaturation, as a sign of acute cardiac failure of different grades of severity.
Palpitations: correlated with arrhythmias or in connection with dyspnea; the feeling of high heart rate or irregular heart rate causes patient discomfort and needs a basal electrocardiogram (ECG) or a cardiac 24-h monitoring to correlate feeling of palpitation with the presence and the severity of arrhythmias.
Chest pain: there are different types of chest pain. It is correlated with pericarditis if it changes during breathing; it is a sign of myocarditis, angina pectoris, or acute coronary syndrome (or aortic dissection) if it is strong and constrictive in the center of the chest and does not change with breaths; the basal ECG during the symptom is extremely useful for the diagnosis. It must be distinguished from esophageal pain that can change during food or water ingestion or osteofibromuscular pain that can change with the touch of the chest or changing the position of the chest.
6.1.2 Signs
All signs are detectable with physical examination:
Lung basal rales, third or fourth heart sounds, and fluid retention if heart failure is present
Irregular heart sounds if arrhythmias, bradycardia, or tachycardia is present
Hypertension or hypotension
6.1.3 Clinic
The clinical scenarios of acute cardiotoxicity are:
Transient dysfunction of left ventricular function, detected with echocardiography, with/without signs of heart failure, myocarditis, pericarditis, and Takotsubo cardiomyopathy
Arrhythmias that can be detected with ECG: ventricular or supraventricular premature complexes, non-sustained supraventricular tachycardia, sinus node dysfunction, and non specific repolarization changes with QTc prolongation
ECG, Doppler echocardiography, and markers of cardiac damage (troponin T and I, natriuretic peptides) can help in the diagnosis.
6.1.4 Anthracycline
Acute clinic toxicity expressions during administration of anthracycline are usually chest pain due to myopericarditis or palpitations due to sinus tachycardia, paroxysmal supraventricular/ventricular tachycardia, and premature atrial and ventricular beats.
Acute left ventricular dysfunction is a rare manifestation but may be reversible (Fig. 6.1).
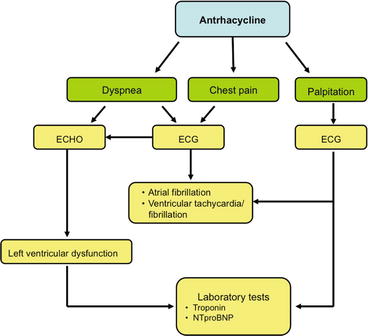

Fig. 6.1
Decision-making flowchart in suspected acute cardiotoxicity from anthracyclines
In addition all anthracyclines can prolong QT (QTc) [4].
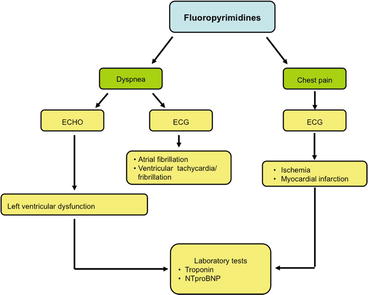
6.1.5 Fluoropyrimidines
6.1.6 Alkylating Agents
The most common acute cardiotoxicity of this group is due to cyclophosphamide that is less potent than etoposide during stem cell mobilization [7]. It appears as a myopericarditis more frequent at high doses, with acute/subacute heart failure usually responding to medical treatment. This event is uncommon (<10 %), transient, and reversible. Palpitations due to supraventricular arrhythmias and atrial fibrillation may occur [8, 9] (Fig. 6.3).
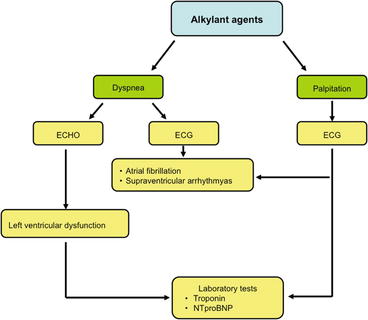

Fig. 6.3
Decision-making flowchart in suspected acute cardiotoxicity from alkylating agents
6.1.7 Platinum Compounds
Acute clinic toxicities during cisplatin infusion are usually palpitations and chest pain due to acute myocardial ischemia or occasionally to myocardial infarction. Dyspnea due to heart failure and electrolyte disturbances may occur [8–10] (Fig. 6.4).
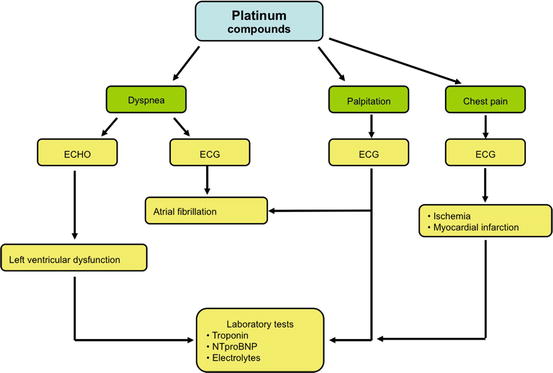

Fig. 6.4
Decision-making flowchart in suspected acute cardiotoxicity from platinum compounds
6.1.8 Antimicrotubule Agents
Most data on cardiac toxicity that have been collected for paclitaxel show uncommon finding (0.5 %) of palpitations due to important cardiac arrhythmias (atrial fibrillation 0.23 %, ventricular tachycardia/fibrillation 0.26 %). This compound may cause dyspnea, dizziness, or syncope due to sinus bradycardia and atrioventricular and bundle branch blocks that however often may be completely asymptomatic [8, 9] (Fig. 6.5).
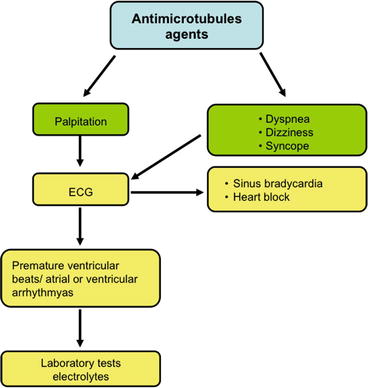

Fig. 6.5
Decision-making flowchart in suspected acute cardiotoxicity from antimicrotubule agents
6.1.9 Perspectives in Cardioprotection from Acute Cardiotoxicity
Only at experimental level, inhibitors of angiotensin II are effective in preventing Adriamycin acute cardiotoxicity preventing ROS formation at intracellular level. In fact, some natural compounds are promising in protecting rat hearts from anthracycline acute myocardial damage: dexrazoxane, schisandrin B [8], ocotillo [9], ginkgo biloba extract [10], and chrysin [11]. Those compounds are promising, but no controlled trials have tested their efficacy in a clinical evidence-based setting.
6.1.10 Treatment
All attempts to prevent acute cardiotoxicity should be done: we expect some effects from certain drugs that are discussed before and we are trying to treat toxicity as soon as possible to avoid permanent damage. If heart failure develops, we should treat it with beta-blockers, ACE inhibitors, and diuretics. If arrhythmias are present, the treatment depends according to the type of rhythm disorder. If the scenario is that of an acute coronary syndrome, we can use guidelines to drive our therapy.
Box 6.1: Acute Cardiotoxicity
Differentiated from hypersensitivity reaction
Common expression: acute heart failure, acute coronary syndrome, arrhythmias hypertension
Main symptoms: dyspnea, chest pain, palpitation
Main signs: signs of heart failure, ECG alterations
Most probable during: anthracycline, fluoropyrimidines, alkylant agents, platinum compounds, antimicrotubules agents
6.2 Chronic Cardiotoxicity
Iris Parrini7
(7)
Cardiology Department, Mauriziano Hospital, Turin, Italy
Late cardiovascular effects are one of the most important complications of cancer therapy. They may be variable in several clinic characters and depend on the type of treatment received. Cardiac complications related to cancer treatment may include cardiomyopathy with or without congestive heart failure, arrhythmias, hypertension, ischemia, and pericardial disease.
In this section possible late complications of a potentially cardiotoxic therapy will be evaluated and related signs and symptom will be analyzed.
In the last decades, cancer therapy has improved the quality of life and survival of the patients, but the late cardiac complications are becoming more and more relevant.
The specificity of these complications depends on the type and on the eventual combination of drugs for the therapy used.
For some treatments as many as one half of patients surviving may develop left ventricular systolic dysfunction with or without heart failure later in life due to the cardiotoxic effects of chemotherapy [12].
In early stages the lack of clinical heart failure may reflect the activation of compensatory mechanisms in the heart and in the body, but when the symptoms develop generally there is a brisk increase in mortality and a significant morbidity [13, 14].
Additionally, radiotherapy induces cardiovascular toxicity in all the cardiac structures: myocardium, pericardium, coronary artery, valve, and conduction tissue.
However, the late effects of anticancer therapy may have many different aspects due to the possibility of the contemporary presence of more than one of these damages and as a result of synergic impact from different therapy.
In clinical practice, appropriate recognition of potential cardiac complications related to chemotherapy or radiotherapy is warranted, but it is possible to have several signs and symptoms of combined cardiac injury that sometimes are not distinguishable from other cardiovascular diseases.
Therefore, in evaluating patients with history of oncological treatment, a close attention to the manifestation of a cardiac disease is needed.
6.2.1 Causes of Late Cardiovascular Effects in Cancer Survivors
In the evaluation of late cardiovascular effects of cancer therapy, it is necessary to collect the history and to perform a deep physical examination to search signs and symptoms of:
Myocardial dysfunction
Systolic dysfunction with or without heart failure symptoms
Diastolic dysfunction with or without heart failure with preserved ejection fraction
Restrictive cardiomyopathy
Myocardial fibrosis
Structural disease
Valve heart disease
Mitral stenosis and insufficiency
Aortic stenosis and insufficiency
Pericardial disease
Conduction system disease
Vascular
Coronary artery disease
Hypertension
Carotid artery disease
Thrombosis
6.2.2 Myocardial Dysfunction
The most common recognized long-term cardiovascular complication of cancer therapy is a chronic cardiomyopathy due to a direct toxicity or sometimes as evolution of ischemic disease.
Another not frequent manifestation is a restrictive cardiomyopathy that represents an advanced stage of myocardial damage with severe diastolic dysfunction.
In clinical practice, several clinic aspects may be present ranging from subclinical manifestation to clear signs and symptoms of heart failure.
Common symptoms are effort dyspnea, orthopnea, and paroxysmal nocturnal dyspnea; less specific symptoms are reduced exercise tolerance and fatigue.
Many specific signs may be present, such as elevated jugular venous pressure and displacement of the apical impulse, third heart sound, gallop rhythm, and cardiac murmurs. Many of the signs of HF as pulmonary congestion and peripheral edema result from sodium and water retention.
6.2.3 Structural Diseases
6.2.3.1 Valve Degeneration
As shown in other chapters about radiation toxicity, mitral and aortic valves are more commonly affected than the right valve. The whole valve apparatus is generally involved. The functional pathologic consequence is more frequently regurgitation than stenosis. When a stenotic lesion occurs, this involves mainly the aortic valve. Patients may be asymptomatic or develop signs and symptoms typically associated with the valve disease and, mainly if there is concomitant myocardial damage, heart failure [15].
6.2.3.2 Pericardial Disease
Pericardial disease develops with or without effusion and can progress to tamponade; pericardial constriction is a rare and a particular late condition in cancer survivors related to radiotherapy [16].
Pericardial effusion may be silent, but when it becomes very large, it results in pericardial tamponade with hemodynamic impairment secondary to the impairment of ventricular filling and reduction of cardiac output.
At first, signs as friction rubs are rare; subsequently, in case of pericardial effusion, heart sounds may completely disappear. If cardiac tamponade develops, common symptoms include dyspnea, orthopnea, hepatomegaly, splenomegaly, ascites, and jugular venous distention. In constrictive pericarditis often the sign of a pericardial knock may be present.
6.2.3.3 Arrhythmias
Rhythm disorders are common both after chemotherapy and radiotherapy and may be functional and transient as premature beats and transient prolongation of QTc interval or may result from direct conduction system damage that leads to tachyarrhythmias or to every degree of cardiac conduction defects, like sick sinus syndrome, bundle branch blocks, and every degree of AV block.
For a schematic approach, see Fig. 6.6.
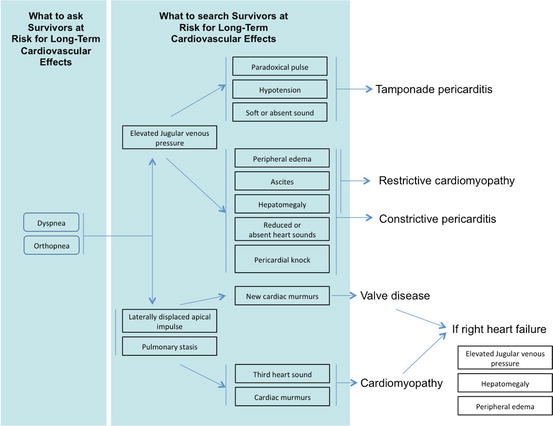

Fig. 6.6
Clinical decision-making for suspected chronic cardiac toxicity from chemotherapy
6.2.4 Vascular Diseases
Atherosclerosis may be induced or accelerated in patients mainly treated with radiotherapy, but not only. Any vascular sites that are involved in the radiation field may develop this consequence. At first, the patient is generally asymptomatic, and then progressive artery damage becomes clinically evident [17].
After chest irradiation patients are at higher risk of development of coronary artery disease. A relative difference with the common atherosclerotic coronary disease is that the vessel involvement is often relatively confined to its origin and can be ostial; moreover, the patients are of younger age. Often symptoms are lacking due to a neuropathy of thoracic nerves that elevates the pain threshold. When symptoms are present the more common expressions are angina, myocardial infarction, and heart failure. However, sudden death may be the first sign of coronary disease in this setting. An appropriate screening with stress tests should be considered in asymptomatic patients with a history of thoracic irradiation.
Additionally, neck irradiation is a major risk factor for carotid disease that may occur asymptomatic or has its onset with an ischemic stroke.
Other vascular events in cancer survivors include hypertension and artery or venous thrombosis.
6.2.4.1 Arterial Hypertension
Hypertension may be a complication of several chemotherapeutic agents in about half of treated subjects [18].
Serious adverse events have been associated with unrecognized and un-managed hypertension. They can be:
1.
Ischemic or hemorrhagic stroke
2.
Heart failure
3.
Myocardial ischemia
4.
Sudden death
Common symptoms are headache, dyspnea, dizziness, or neurologic symptoms.
6.2.4.2 Arterial and Venous Thrombosis
Arterial or venous thrombosis can be due to many causes correlated to the cancer itself that can lead to a pro-thrombotic situation or to the treatment itself. This condition may overlap to an atherosclerotic plaque leading to an increased possibility of thrombosis and to complications like myocardial infarction.
From a practical point of view, see Fig. 6.7.
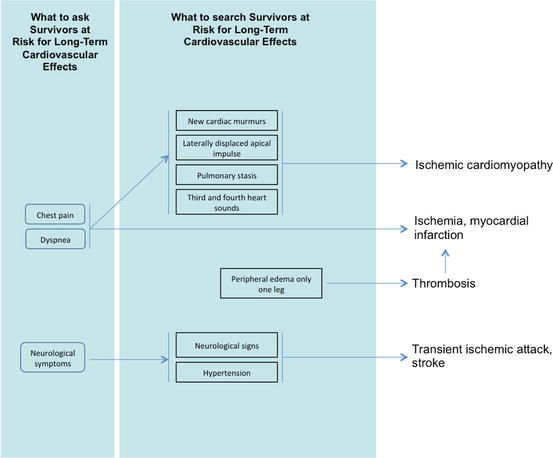

Fig. 6.7
Clinical decision-making for long-term suspected cardiotoxicity from chemotherapy
Box 6.2: Chronic Cardiotoxicity
Commonest expression of cardiotoxicity from cancer therapy
Often symptoms clear only after years from therapy
Myocardial dysfunction (systolic, diastolic, restrictive), structural dysfunctions (valve, pericardial, conduction system), vascular dysfunctions (coronary artery, carotid artery hypertension, thrombosis)
6.3 Systolic Dysfunction
Iris Parrini8
(8)
Cardiology Department, Mauriziano Hospital, Turin, Italy
Cardiac dysfunction can be due to several chemotherapeutic agents and the onset timing is often variable. Echocardiography is the most widely used method of evaluation; with the addition of global longitudinal strain (GLS) examination, it is possible to detect early systolic dysfunction.
CMR is the gold standard in the evaluation of left ventricular dysfunction. Its main limitation is the cost and should be reserved to the patients in whom echocardiographic quality is not optimal.
One of the most frequent cardiovascular complications of chemotherapy and radiotherapy is cardiomyopathy, which may become clinically relevant even years after treatment.
The most important information comes from the pediatric population cancer survivors having a long life expectancy. In adults the development may be multifactorial and general cardiovascular risk factors have to be added to the effect of the anticancer therapy, with a net balance difficult to evaluate.
It is well known how several chemotherapy agents may lead to the development of LV dysfunction [19]. Anthracyclines play a major role among the anticancer drugs in use, and their cardiotoxic effects have been studied from many years [20, 21]. Other classes have also been identified to cause significant cardiac toxicity, including alkylating agents, tyrosine kinase inhibitors, antimicrotubule agents, and monoclonal antibody as shown in the previous chapters.
The cardiotoxic expression of systolic dysfunction may range from subclinical damage to signs and symptoms of overt heart failure.
At baseline the clinical approach consists of the following:
Knowledge of chemotherapeutic agents that can lead to systolic dysfunction.
Evaluate the presence of cardiovascular risk factors for heart disease that can be used in combination to stratify the risk of cardiotoxicity.
Baseline assessment including an ECG to detect ischemia or arrhythmias, echocardiogram for evaluation of the cardiac function (when it is possible perform a GLS), and blood sample with troponin and natriuretic peptides.
When LVEF is <53 % before the start of the therapy, a consultation with a cardiologist is recommended.
During chemotherapy the clinical approach is as follows:
Monitor LVEF with echocardiogram (when it is possible perform a GLS).
Blood sample with troponin and natriuretic peptides.
If LVEF is reduced, follow the directions shown in previous paragraphs. If possible perform GLS: a decline of 10 % points to an LVEF < 53 % or a decrease of >15 % compared with baseline GLS is recommended a cardiology consultation due to evidence of subclinical dysfunction.
Begin cardioprotective therapy.
After conclusion of chemotherapy, the clinical approach is as follows:
Clinical examination to look for signs and symptoms of heart failure.
If confirmed check blood tests and cardiac imaging to evaluate the severity of the systolic dysfunction and begin therapy according to the heart failure guidelines [22].
For a schematic clinical point of view, see Fig. 6.8.
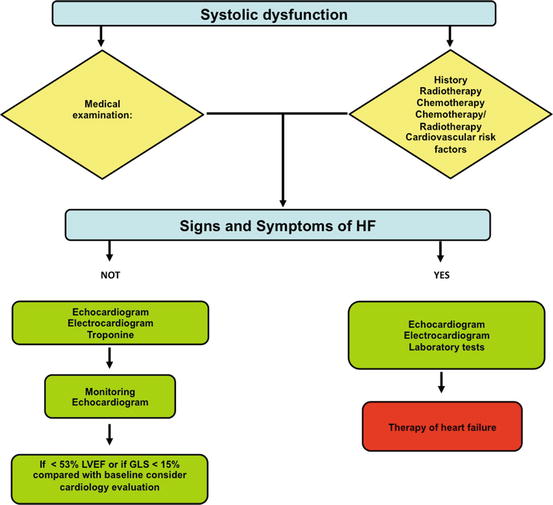

Fig. 6.8
Diagnostic flowchart for suspected systolic dysfunction
Many cardiac imaging tools may be helpful at baseline and during and after the chemotherapy for the diagnosis of cardiac damage.
ECG may add additional diagnostic information in detecting cardiotoxicity because it is sometimes associated with rhythm disturbance, heart block, or ischemic changes.
Echocardiography is a widely used method for the measurement of LVEF, but it is limited by suboptimal image and inter- and intra-observer variability [19, 20].
Diastolic dysfunction detected by Doppler pulsed analysis and DTI often precedes alterations of systolic dysfunction but has no predictive value later [21].
Global longitudinal strain is a parameter for early detection of systolic dysfunction and is predictive of late LVEF decrease [23].
Radionuclide ventriculography or multiple-gated acquisition scan (MUGA) has been validated as an accurate and reproducible method for LVEF estimation, but exposes patients to ionizing radiation [24, 25].
Cardiac MRI is considered the gold standard for volume and LVEF determination¸ but the higher costs limit its use. MRI has to be considered when measure of LVEF is controversial or quality image of echocardiography is inadequate [26].
Nowadays, the effort of cardiologists and oncologists is to find out early systolic dysfunction improving the survival of cancer patients especially for systolic dysfunction [27].
Box 6.3: Systolic Dysfunction
Due to several chemotherapy agents and radiotherapy
Clinic onset timing variable
Clinic approach:
Knowledge of possible toxicity of the chemotherapy agent
Evaluation of risk factors for toxicity
Baseline evaluation of ECG, ECHO, biomarkers
If baseline LCEF < 53% cardiac consultation before start therapy
During therapy careful monitoring
Eventually stop or modify chemotherapy and/or begin cardioprotective therapy
Long term follow-up
6.4 Hypertension: How to Evaluate, How to Prevent, and How to Treat
Gonzalo Baron Esquivias9 , Alessandro Bonzano10, Iris Parrini11 and Xavier Garcia-Moll12
(9)
Cardiology Department, Hospital Universitario Virgen del Rocio, Sevilla, Spain
(10)
Cardiology Unit, Service Department, IRCCS Candiolo – Turin, Turin, Italy
(11)
Cardiology Department, Mauriziano Hospital, Turin, Italy
(12)
Cardiology Department, Hospital de la Santa Creu i Sant Pau, University Hospital, Barcelona, Spain
High levels of arterial pressure are common in cancer patients treated with chemotherapy. Hypertension may lead to severe complications per se or may aggravate other complications of cancer therapy.
To avoid further complications it is of paramount relevance to identify and treat the subjects that may be at risk in this condition.
Common treatment of hypertension is used also in this clinical setting, but it has to be particularly intensive and controlled.
Every coronary risk factor has a powerful impact on prognosis of patients with cancer treated with chemotherapy or radiotherapy. Hypertension is one of the most important of these risk factors. This can be a preexisting condition or may also develop during some specific chemotherapy. It is of paramount relevance to treat actively the first condition and to prevent the second.
The high prevalence of hypertension across the world implies that several cancer patients, especially those at older ages, have essential hypertension at the time of diagnosis of cancer; in fact hypertension is the most common comorbidity in patients with malignancy (37 %) [28]. But the relation between cancer and hypertension is not only a coincidence; there are four aspects involved in that relation.
Firstly, in 1975, Dyer et al. reported a positive association between high blood pressure and cancer mortality in a cohort of 1,233 Chicago gas company male employees [29]. Furthermore, there are literature references supporting a link between hypertension and cancer; however, a mechanism for this association has not been defined. A meta-analysis including a very large prospective sample size from seven European population-based cohorts with virtually complete capture of cancer cases showed that elevated BP was statistically significantly associated with cancer incidence in men and with cancer mortality in men and women, as well as with several specific cancers. In that study, cancer risk increased linearly with increasing BP levels, and for both cancer incidence and mortality, the association was stronger for men than for women. Among men, the absolute 20-year risk of cancer incidence or mortality at age 50 years was 1–2 % points higher with hypertensive systolic or diastolic BPs compared with men with normal BP [30].
Secondly, the relation of hypertension and cancer with risk factors is real. Salt intake contributes to hypertension and is implicated as a causal factor in gastric cancer. Analogously, heavy alcohol consumption contributes to both increased blood pressure and many malignancies. Another powerful risk factor for hypertension is obesity, reinforced by a Swedish study that found independent dose–response relations between body mass index and the risk of renal-cell cancer and between diastolic and systolic blood pressure and the risk of renal-cell cancer, with a greater risk observed in men with an even slightly higher body mass index or blood pressure than in their counterparts with lower values [31]. Because increased consumption of fat results in an increase in both weight and blood pressure and has also been implicated as a risk factor for some forms of cancer, dietary fat may explain the correlation between blood pressure and cancer. Similarly, low socioeconomic status simultaneously confers an increased risk of high blood pressure, certain cancers, and exposure to known carcinogens (e.g., alcohol and tobacco).
Thirdly, blood pressure regulation is modulated not only by risk factors but also by another key players that include the dopamine-1 receptor (D1R), G protein-coupled kinase type 4 (GRK4), and c-Myc, all found in renal proximal tubule cells. Some of them play a role in cancer development, such as c-Myc that also regulates GRK4 in the breast cancer cell line MCF7.
Finally, hypertension prevalence before chemotherapy is around 30 %, and that is similar in the general population, but there is a relation between hypertension and cancer treatment. HT is a recognized effect of antimetabolite purine analogs such as clofarabine; natural agent vinca alkaloids such as vinblastine and vincristine; taxanes such as paclitaxel and docetaxel; anti-small molecule tyrosine kinase such as dasatinib, imatinib, lapatinib, sorafenib, sunitinib, and pazopanib; monoclonal antibody such as bevacizumab; hormones such as prednisone; and antiandrogens such as abiraterone acetate [32, 33]. Table 6.2 shows the most described prevalence [34–42].
Table 6.2
Hypertension incidence in clinical trials of vascular endothelial growth factor inhibitors
Agent | First author, year (reference) | Total incidence (%) |
|---|---|---|
Aflibercept | Ten, 2007 [34] | 46 |
Axitinib | Qi, 2013 [35] | 40.1 |
Bevacizumab | Hurwitz, 2004 [36] | 22 |
Cediranib | Robinson, 2010 [37] | 67 |
Motesanib | Sherman, 2008 [38] | 56 |
Pazopanib | Qi, 2013 [39] | 35.9 |
Sorafenib | Wu, 2008 [40] | 23.4 |
Sunitinib | Motzer, 2007 [41] | 24 |
Vandetanib | Qi, 2013 [42] | 24.2 |
The association between cancer and hypertension is a growing problem considering the high prevalence of both conditions. Among chemotherapeutic drugs, anti-VEGFs are the most frequently involved in a rise of blood pressure levels, mainly through the decrease in NO synthesis. Regarding the relationship between antihypertensive agents and risk of cancer development, many studies have been conducted, leading to conflicting results. However, the most recent studies have denied an increased risk of cancer in patients receiving antihypertensive drugs [43]. The control of a de-novo hypertension or worsened pre-existing hypertension control after initiation of an anticancer treatment may indicate many possible underlying mechanisms: endothelial dysfunction associated with decreased bioavailability of nitric oxide and increased endothelin production vascular and kidney, increased vascular tone, vascular rarefaction (decreased microvessel density), and glomerular renal thrombotic microangiopathy with high structural and functional changes that lead to hypertension and proteinuria, glomerular lesions, or more often, secondary concerned itself hypertension treatment. In the absence of a single dominant mechanism, most of them are usually present [44–46]. In almost all cases, glomerular or vascular involvement or worsening of previous damages explains the appearance of hypertension and hence the importance of assessing renal abnormalities (creatinine clearance, proteinuria, hematuria) and markers of hemolytic anemia or thrombocytopenia.
6.4.1 Prevention
The association between the two conditions requires cardiologists and oncologists to develop joint strategies to walk in both directions. The objective is both to reduce the impact that the hypertension can be in the oncologic pathology and in the opposite direction the impact that cancer and its treatment may have on hypertension. Both professionals should approach the cancer patient and be working together, just as the heart team named in other pathologies.
Patients with cancer and major coronary risk factors need treatment of all modifiable risk factors (Table 6.3).
Table 6.3
Coronary risk factors
Hypertension |
Cigarette smoke |
Diabetes |
Hypercholesterolemia LDL |
Family history of CAD |
Metabolic syndrome |
The first step in the evaluation regarding hypertension consists in the necessity to search for risk factors for hypertension (Table 6.4).
Table 6.4
Risk factors for hypertension
Genetic predisposition or family history |
Black race |
Diagnosis of prehypertension |
Increasing age |
Obesity |
High sodium–low potassium intake |
Excessive alcohol intake |
Low socioeconomic status |
Sleep apnea |
Use of illegal drugs (cocaine) or over-the-counter medications |
6.4.2 Patient Management (Fig. 6.9)
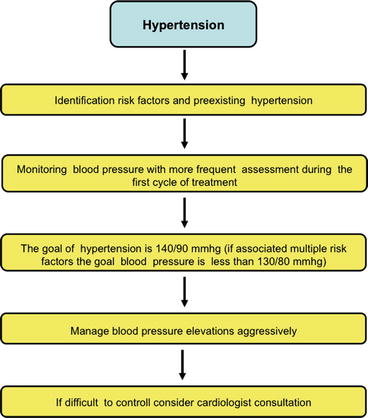
Fig. 6.9
Diagnostic flowchart for hypertension
The recognition of cardiovascular risk factors (Tables 6.2 and 6.3) and specially preexisting hypertension is mandatory in cancer patients before starting the therapy.
It is necessary to perform a complete history and objective evaluation of the patient regarding both diseases; in regard with hypertension it is necessary to know the hemodynamical situation, the heart and vessel repercussion, treatment effectiveness, and possible interactions. In regard with cancer, it is mandatory to know the possible relationship between cancer type and hypertension, cancer status, and oncology therapy.
When evaluating the management of hypertension in an oncology patient, it is mandatory to establish the same premises as any other patient with hypertension. A consensus document published in 2010 sets out five recommendations that can be summarized useful not only for cancer patients receiving a specific treatment with inhibitors but for all oncology patients with hypertension [32]: (1) There is a need for patient risk stratification at baseline – preexisting hypertension is common in cancer patients and should be identified and treated before initiation therapy. (2) Active monitoring of blood pressure throughout the period of cancer management is recommended, especially during the first cycle of therapy when most patients experience a secondary elevation in blood pressure. (3) The target of blood pressure management should match the JNC 7 classification and European guidelines with the target blood pressure below 140/90 mmHg [47]. (4) Aggressive blood pressure control is advised in order to minimize the risk of end-organ damage. (5) Attention to the choice of antihypertensive medication is also recommended. The panel encourages the referral of patients to hypertension specialists whenever oncologists face difficulties in achieving adequate blood pressure control.
6.4.3 Cancer Drugs and Hypertension
Table 6.5 shows what chemotherapy drugs are involved in the development of hypertension.
Table 6.5
Hypertension and antineoplastic drugs
Name | Indications | Heart effects | HTN | Kidney damage | Other toxicity | Interaction with antihypertensive drugs | Hepatic failure correction/renal failure correction |
|---|---|---|---|---|---|---|---|
Antimetabolites: purine analogs | |||||||
Clofarabine | Lymphatic Acute leukemia | 27,00 | Yes | Yes | Myelotoxicity Hepatotoxicity Nephrotoxicity | No | Yes (red. dose) Yes (red. dose) |
Natural agents: vinca alkaloids | |||||||
Vinblastine | Hodgkin/non-Hodgkin lymphomas Testicular cancer Kaposi’s sarcoma Breast cancer Mycosis fungoides | Yes | Yes | No | Myelotoxicity Gastrointestinal toxicity Thrombocytopenia | Diltiazem Verapamil Felodipine Nifedipine (Increase of vinblastine) | Yes (↓dose)/no |
Vincristine | Hodgkin/non-Hodgkin lymphomas Rhabdomyosarcoma Leukemia | Yes | Yes | No | Myelotoxicity Neurotoxicity | Diltiazem Verapamil Felodipine Nifedipine | Yes (↓dose)/no |
Natural agents: taxanes | |||||||
Paclitaxel | Ovarian cancer Metastatic breast cancer Non-small cell lung cancer Kaposi’s sarcoma | 2.3–8 % | Yes | No | Myelotoxicity Neurotoxicity Gastrointestinal/skin/liver toxicity | Diltiazem Verapamil Felodipine Nifedipine | Yes (↓dose)/ND |
Docetaxel | Metastatic breast cancer Non-small cell lung cancer Prostatic cancer Gastric cancer Head and neck cancer | 2.3–8 % | Yes | No | Anemia Thrombocytopenia Neutropenia Leucopenia Hepatotoxicity Arrhythmias | Diltiazem Verapamil Felodipine Nifedipine < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |