6 Special Problems
I. CONGESTIVE HEART FAILURE
A. CAUSES
1. Congenital heart disease. Volume overload lesions such as ventricular septal defect (VSD), patent ductus arteriosus (PDA), and endocardial cushion defect (ECD) are the most common causes of CHF in the first 6 months of life. In infancy the time of the onset of CHF varies predictably with the type of defect. Table 6-1 lists common defects according to the age at which CHF develops. Large left-to-right shunt (L-R shunt) lesions, such as VSD and PDA, do not cause CHF before 6 to 8 weeks of age because the pulmonary vascular resistance (PVR) does not fall low enough to cause a large shunt until this age. CHF may occur earlier in premature infants (within the first month) because of an earlier fall in the PVR. Note that children with tetralogy of Fallot (TOF) do not develop CHF and that children with atrial septal defect (ASD) rarely develop CHF in the pediatric age group, although ASD causes CHF in adulthood.
2. Acquired heart disease. Acquired heart disease of various etiologies can lead to CHF. Common entities (with the approximate time of onset of CHF) are as follows:
TABLE 6-1 CAUSES OF CONGESTIVE HEART FAILURE DUE TO CONGENITAL HEART DISEASE ACCORDING TO THE TIME OF OCCURRENCE
| TIME OF OCCURRENCE | CAUSES |
|---|---|
| At birth | Hypoplastic left heart syndrome (HLHS) |
| Volume overload lesions (e.g., severe TR or PR, large systemic AV fistula) | |
| First week | Transposition of the great arteries (TGA) |
| PDA in small premature infants | |
| HLHS (with more favorable anatomy) | |
| TAPVR, particularly those with pulmonary venous obstruction | |
| Critical AS or PS | |
| Others: systemic AV fistula | |
| 1–4 weeks | COA (with associated anomalies) |
| Critical AS | |
| Large L-R shunt lesion (e.g., VSD, PDA) in premature infants | |
| All other lesions listed above | |
| 4–6 weeks | Some L-R shunt lesions, such as ECD |
| 6 weeks-4 months | Large VSD |
| Large PDA | |
| Others: anomalous left coronary artery from the PA |
ECD, endocardial cushion defect.
B. CLINICAL MANIFESTATIONS
Plasma levels of natriuretic peptides, atrial natriuretic peptide (ANP), and B-type natriuretic peptide (BNP) are increased in most adult patients with dyspnea from heart failure but not in dyspnea caused by pulmonary disease. Plasma levels of these peptides are normally elevated in the first weeks of life. Although increased levels of BNT and the N-terminal segment of its pro-hormone (NT-ProBNT) have been reported in most children with CHF, the usefulness of data on the levels of these peptides appears limited because an appropriate reference range has not been established. The levels of these peptides are different depending on the commercial testing kits used.
1. Poor feeding of recent onset, tachypnea, poor weight gain, and cold sweat on the forehead suggest CHF in infants. In older children, shortness of breath, especially with activities; easy fatigability; puffy eyelids; or swollen feet may be presenting complaints.
2. Physical findings can be divided by pathophysiologic subgroups.
3. Cardiomegaly on CXR films is almost always present, except when the pulmonary venous return is obstructed; in that case pulmonary edema or venous congestion will be present.
4. The ECG is not helpful in deciding whether one is in CHF, although it may be helpful in determining the cause.
5. Echo studies confirm the presence of chamber enlargement or impaired left ventricle (LV) function and help determine the cause of CHF.
C. MANAGEMENT
1. Treatment of underlying causes or contributing factors
2. General measures. Nutritional supports are important. Infants in CHF need significantly higher caloric intakes than recommended for average children. The required calorie intake may be as high as 150 to 160 kcal/kg/day for infants in CHF.
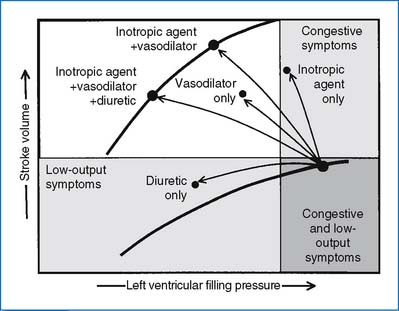
3. Drug therapy. Three major classes of drugs are commonly used in the treatment of CHF in children: diuretics, inotropic agents, and afterload-reducing agents.
4. Surgical management. If medical treatment as outlined previously does not improve CHF caused by CHD within a few weeks to months, one should consider either palliative or corrective cardiac surgery for the underlying cardiac defect when technically feasible. Cardiac transplantation is an option for a patient with progressively deteriorating cardiomyopathy despite maximal medical treatment.
BOX 6-1 METHODS OF INCREASING CALORIC DENSITY OF FEEDINGS
1. Human milk fortifier (Enfamil, Mead Johnson), 1 packet per 25 mL of breast milk ≡ 24 kcal/oz
2. Formula concentration to 24 kcal/oz by:
3. Supplementation of formula to 26–30 kcal/oz is accomplished in the following manner.
4. Pediasure (Ross), 30 kcal/oz ready-to-feed (for children over 1 year of age)
From Wright GE, Rochini AP: Primary and general care of the child with congenital heart disease, ACC Current Journal Review 89–93, March/April 2002.
TABLE 6-2 DIURETIC AGENTS AND DOSAGES
| PREPARATION | ROUTE | DOSAGE |
|---|---|---|
| Thiazide Diuretics | ||
| Chlorothiazide (Diuril) | Oral | 20–40 mg/kg/day in two divided doses |
| Hydrochlorothiazide (HydroDIURIL) | Oral | 2–4 mg/kg/day in two divided doses |
| Loop Diuretics | ||
| Furosemide (Lasix) | IV | 1 mg/kg/dose |
| Oral | 2–3 mg/kg/day in two or three divided doses | |
| Ethacrynic acid (Edecrin) | IV | 1 mg/kg/dose |
| Oral | 2–3 mg/kg/day in two or three divided doses | |
| Aldosterone Antagonists | ||
| Spironolactone (Aldactone) | Oral | 3 mg/kg/day in two or three divided doses |
TABLE 6-3 SUGGESTED DOSAGES OF RAPID-ACTING CATECHOLAMINES
| DRUG | ROUTE AND DOSAGE | SIDE EFFECTS |
|---|---|---|
| Epinephrine (Adrenalin) | IV 0.1–1 μg/kg/min | Hypertension, arrhythmias |
| Isoproterenol (Isuprel) | IV 0.1–0.5 μg/kg/min | Peripheral and pulmonary vasodilation |
| Dobutamine (Dobutrex) | IV 5–8 μg/kg/min | Little tachycardia and vasodilation, arrhythmias |
| Dopamine (Inotropin) | IV 5–10 μg/kg/min | Tachycardia, arrhythmias, hypertension, or hypotension |
| Dose-related cardiovascular effects of dopamine (μg/kg/min): | ||
| Renal vasodilation (2–5) | ||
| Cardiac (5–15) | ||
| Vasoconstriction (15–20) |
TABLE 6-4 ORAL DIGOXIN DOSAGE FOR CONGESTIVE HEART FAILURE
| PATIENT | TDD* (μG/KG) | MAINTENANCE*† (μG/KG/DAY) |
|---|---|---|
| Premature babies | 20 | 5 |
| Neonates | 30 | 8 |
| Less than 2 years | 40–50 | 10–12 |
| More than 2 years | 30–40 | 8–10 |
* IV dose is 75% of the oral dose.
† Maintenance dose is 25% of the TDD in two divided doses. TDD, total digitalizing dose.
From Park MK: The use of digoxin in infants and children with specific emphasis on dosage, J Pediatr 108:871–877, 1986.
BOX 6-2 FACTORS THAT MAY PREDISPOSE TO DIGITALIS TOXICITY
HIGH SERUM DIGOXIN LEVEL
High-dose requirement, as in treatment of certain arrhythmias
Decreased renal excretion (premature infants, renal disease)
BOX 6-3 ECG CHANGES ASSOCIATED WITH DIGITALIS
EFFECTS
Shortening of QTc is the earliest sign of digitalis effect
Sagging ST segment and diminished amplitude of T wave (T vector does not change)
TABLE 6-5 DOSAGES OF VASODILATORS
| DRUG | ROUTE AND DOSAGE | COMMENTS |
|---|---|---|
| Arteriolar Vasodilators | ||
| Hydralazine (Apresoline) | ||
| Venodilators | ||
| Nitroglycerin | IV: 0.5–1 μg/kg/min (maximum 6 μg/kg/min) | Start with small dose and titrate based on effects |
| Mixed Vasodilators | ||
| Captopril (Capoten) | ||
| Enalapril (Vasotec) | Oral: 0.1 mg/kg, once or twice daily | Patient may develop hypotension, dizziness, or syncope |
| Nitroprusside (Nipride) | IV: 0.3–0.5 μg/kg/min; titrate to effects (max dose 10 μg/kg/min) | May cause thiocyanate or cyanide toxicity (e.g., fatigue, nausea, disorientation), hepatic dysfunction, or light sensitivity |
II. CHILD WITH CHEST PAIN
A. CAUSE AND PREVALENCE
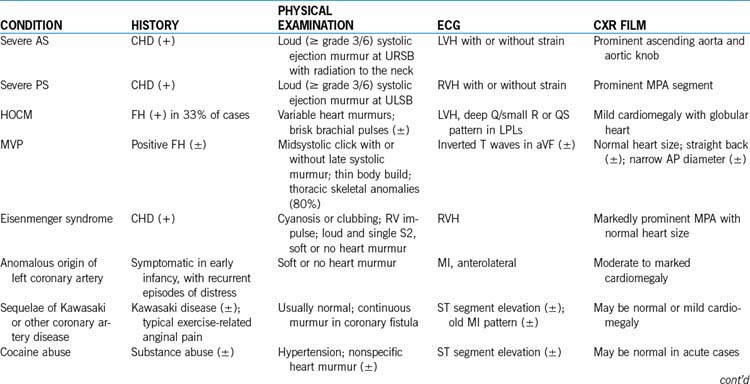
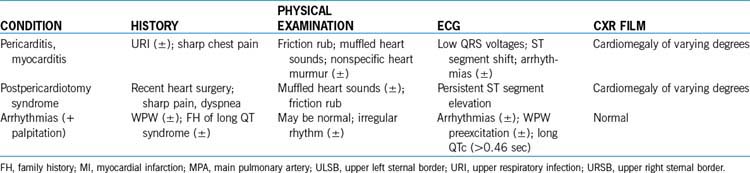
Table 6-6 lists the frequency of the causes of chest pain in children according to organ systems. The three most common causes of chest pain in children are costochondritis, trauma to or muscle strain of the chest wall, and respiratory diseases, especially those associated with coughing. These three conditions account for 45% to 65% of cases of chest pain in children. Chest pain of cardiac origin occurs in only 0% to 4% of children with complaint of chest pain. Box 6-4 is a partial list of possible causes of noncardiac and cardiac chest pain in children. Psychogenic causes are less likely found in children younger than 12 years old; such causes are more likely to be found in females older than 12 years of age.
TABLE 6-6 FREQUENCY OF CAUSES OF CHEST PAIN IN CHILDREN
| CAUSE | INCIDENCE (%) |
|---|---|
| Idiopathic | 12–45 |
| Costochondritis | 9–22 |
| Musculoskeletal trauma | 21 |
| Cough, asthma, pneumonia | 15–21 |
| Psychogenic | 5–9 |
| Gastrointestinal system | 4–7 |
| Cardiac disorder | 0–4 |
| Sickle cell crisis | 2 |
| Miscellaneous | 9–21 |
BOX 6-4 CAUSES OF CHEST PAIN IN CHILDREN AND ADOLESCENTS
NONCARDIAC CAUSES
B. CLINICAL MANIFESTATIONS
1. Idiopathic chest pain. No cause can be found in 12% to 45% of patients, even after a moderately extensive investigation. In children with chronic chest pain a cardiac cause is less likely to be found.
2. Noncardiac causes of chest pain. Identifiable noncardiac causes of chest pain are found in 56% to 86% of reported cases, most often in the thorax and respiratory system (Table 6-6).
3. Cardiac causes of chest pain. Cardiac chest pain may be caused by ischemic ventricular dysfunction, pericardial or myocardial inflammatory processes, or arrhythmias, and these cardiac causes occur in 0% to 4% of cases (Box 6-4). Table 6-7 summarizes important clinical findings of cardiac causes of chest pain in children.
C. DIAGNOSTIC APPROACH
1. History of present illness. The initial history is directed at determining whether the pain is likely of cardiac origin. One asks about the nature of the pain, in terms of its association with exertion or physical activities, the intensity, character, frequency, duration, and points of radiation. A typical anginal pain is located in the precordial or substernal area and radiates to the neck, jaw, either or both arms, back, or abdomen. Exercise, heavy physical activities, or emotional stress typically precipitate the pain. It is not a sharp pain. The patient describes the pain as a deep, heavy pressure; the feeling of choking; or a squeezing sensation. Nonexertional sharp pain of short duration is usually not of cardiac origin. Associated symptoms such as syncope, dizziness, or palpitation with chest pain suggest potential cardiac origin of the pain. Pain that changes with position of the body may suggest pleural pain. The following are some examples of questions used in determining the nature of chest pain.
4. Other investigations. CXR films (for pulmonary pathology, cardiac size and silhouette, and pulmonary vascularity) and an ECG (for arrhythmias, hypertrophy, conduction disturbances, Wolff-Parkinson-White [WPW] preexcitation, and prolonged QT intervals) may be obtained. Drug screening is ordered when cocaine-induced chest pain is suspected. Clinical findings of cardiac causes of chest pain are summarized in Table 6-7.
5. Tentative diagnosis of noncardiac chest pain
6. Referral to cardiologists. The following are some indications for referral to a cardiologist for cardiac evaluation of chest pain.
D. TREATMENT
1. Costochondritis can be treated by reassurance and occasionally by nonsteroidal antiinflammatory agents (such as ibuprofen) or acetaminophen. Ibuprofen is a better choice because it is an antiinflammatory as well as analgesic agent.
2. Most musculoskeletal and nonorganic causes of chest pain can be treated with rest, acetaminophen, or nonsteroidal antiinflammatory agents.
3. Exercise-induced asthma is most effectively prevented by inhalation of a β2-agonist immediately before exercise. Inhaled albuterol usually affords protection for 4 hours.
4. If gastritis, gastroesophageal reflux, or peptic ulcer disease is suspected, trials of antacids, hydrogen ion blockers, or prokinetic agents ( such as metoclopramide [Reglan]) are helpful therapeutically (as well as diagnostically).
5. If organic causes of chest pain are not found and psychogenic etiology is suspected, psychological consultation may be considered.
6. The correct therapy for acute cocaine toxicity has not been established. Calcium channel blockers (nifedipine, nitrendipine), β-adrenergic blockers, nitrates, and thrombolytic agents have resulted in varying levels of success. The use of β-blockers is controversial; they may worsen coronary blood flow.
III. SYNCOPE
C. CAUSES
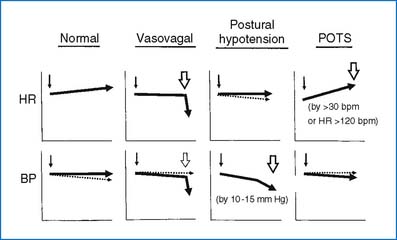
The normal function of the brain depends on a constant supply of oxygen and glucose. Significant alterations in the supply of oxygen and glucose may result in a transient loss or near loss of consciousness. Syncope may be due to noncardiac causes (usually autonomic dysfunction), cardiac conditions, neuropsychiatric, and metabolic disorders. Box 6-5 lists possible causes of syncope.
BOX 6-5 CAUSES OF SYNCOPE
AUTONOMIC (NONCARDIAC)
Vasovagal syncope (also known as simple, neurocardiogenic, or neurally mediated syncope)
Orthostatic (postural) hypotension (dysautonomia)
Exercise-related syncope (see further discussion in text)
CARDIAC
Tachycardias: SVT, atrial flutter/fibrillation, ventricular tachycardia (seen with long QT syndrome, arrhythmogenic RV dysplasia)
Bradycardias: sinus bradycardia, asystole, complete heart block, pacemaker malfunction
1. Noncardiac causes of syncope
2. Cardiac causes of syncope. Cardiac causes of syncope may include obstructive lesions; myocardial dysfunction; and arrhythmias, including long QT syndrome.
D. EVALUATION OF A CHILD WITH SYNCOPE
1. History. Accurate history taking is most important in determining cost-effective diagnostic strategies.
2. Physical examination. Although physical examination is usually normal, it should always be performed, focusing on the cardiac and neurologic systems.
3. Diagnostic studies. History and physical examinations guide practitioners in choosing the diagnostic tests that apply to a given syncopal patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree